Although widespread, actions aiming at the restoration of native species populations within their indigenous range still lack a clear definition of success, given the high degree of variability in species needs. In this sense, to understand and manage the mechanisms that lead to reintroduction or reinforcement failures may be a more feasible alternative to ensure conservation objectives. In this study, we aimed to systematize the main drivers that can negatively impact bird population restoration according to researchers and practitioners. Thus, a systematic review was performed in peer-reviewed journals, identifying 75 attempts, conducted from 1990 to 2016, in 30 countries involving 64 bird species and subspecies. Thirteen drivers that negatively impact reintroduction or reinforcement attempts were identified, where predation, unexpected dispersal movement and diseases were the main factors. We believe that if these drivers were prioritized during pre-release planning and post-release monitoring, restoration population programs would be more successful.
The IUCN Red List process has been globally applied to reveal the threat degree of species and ecosystems (Mace et al., 2008; IUCN, 2015; Rodríguez et al., 2015). To reverse or even mitigate the threat degree, different conservation strategies have been executed (Tulloch et al., 2015), and population restoration stands out as one of the most widespread (Soorae, 2013). According to the IUCN (2013), population restoration is any intentional movement (translocation) and release of a living organism to within its indigenous range. It comprises two activities: reinforcement and reintroduction, that differ in the presence or absence of conspecific populations before release, and not specifically in management techniques (IUCN, 2013; Seddon et al., 2014). Reinforcement, also known as augmentation, supplementation, re-stocking, or enhancement (plants only), is the release of an organism into an existing population of conspecifics (IUCN, 2013; Hardouin et al., 2014), aiming to enhance population viability by increasing population size, genetic diversity, or representation of specific demographic groups or stages (Bretagnolle and Inchausti, 2005; Champagnon et al., 2012; IUCN, 2013). Reintroduction, on the other hand, is the release of an organism inside the indigenous range from which it has disappeared (Armstrong and Seddon, 2007; IUCN, 2013). Its main objective is to re-establish a viable population of the focal species within its indigenous range, fulfilling a role as a keystone component of an ecosystem, and/or create the public and political support necessary to undertake habitat restoration or to put species protection measures in place (Seddon, 1999; Lipsey and Child, 2007). However, while conceptually well established, there is no consensus on how to measure the success of reintroduction or reinforcement efforts (Seddon, 1999; Haskins, 2015; Robert et al., 2015).
Several methodological proposals to evaluate population restoration are available worldwide (Soorae, 2013). As a basic metric of success, some authors consider first-year survival rates within the normal range reported for avian fledglings to be indicative of a successful release (White et al., 2005). In other studies, researchers regard survival and reproduction as the two most fundamental parameters in terms of population establishment and persistence, defining ‘success’ as those translocations in which first-year survival was >0.50 (i.e. survival>mortality) and in which released birds later bred with conspecifics, either captive-reared or wild (White et al., 2012). Moreover, other authors also believe that three objectives should be achieved in an effort to restore a population: (i) establishment: the survival of the release generation; (ii) growth: breeding by the release generation and their offspring; and, (iii) regulation: persistence of the re-established population (Seddon, 1999; Sarrazin, 2007; Miller et al., 2014). For these authors, although the establishment and growth phases are necessary for success, they do not provide accurate estimates of the long-term viability of a reintroduced population. Thus, the ultimate success criteria should focus on the regulation phase, during which population dynamics critically depend on the interactions among species and habitat characteristics, in order to draw reliable conclusions about long-term population dynamics (Armstrong and Reynolds, 2012).
To contribute to the development of the science of reintroduction biology, Robert et al. (2015) proposed a method that assesses if the viability of reintroduced populations could be evaluated using the same criteria as for remnant populations, such as the International Union for Conservation of Nature (IUCN) Red List criteria. For this, two postulates were proposed: (i) that successful reintroduction programs should produce viable populations and (ii) that reliable assessments of ultimate success require that populations reach their regulation phase (Robert et al., 2015). However, Haskins (2015) point out fragilities in this methodology, since the time and resources required cannot keep pace with the ever-growing demand for conservation action, particularly under a rapidly changing climate, and the standardized definition of reintroduction success is nearly impossible to obtain, due to the high degree of variability in species needs when it comes to reintroduction success criteria.
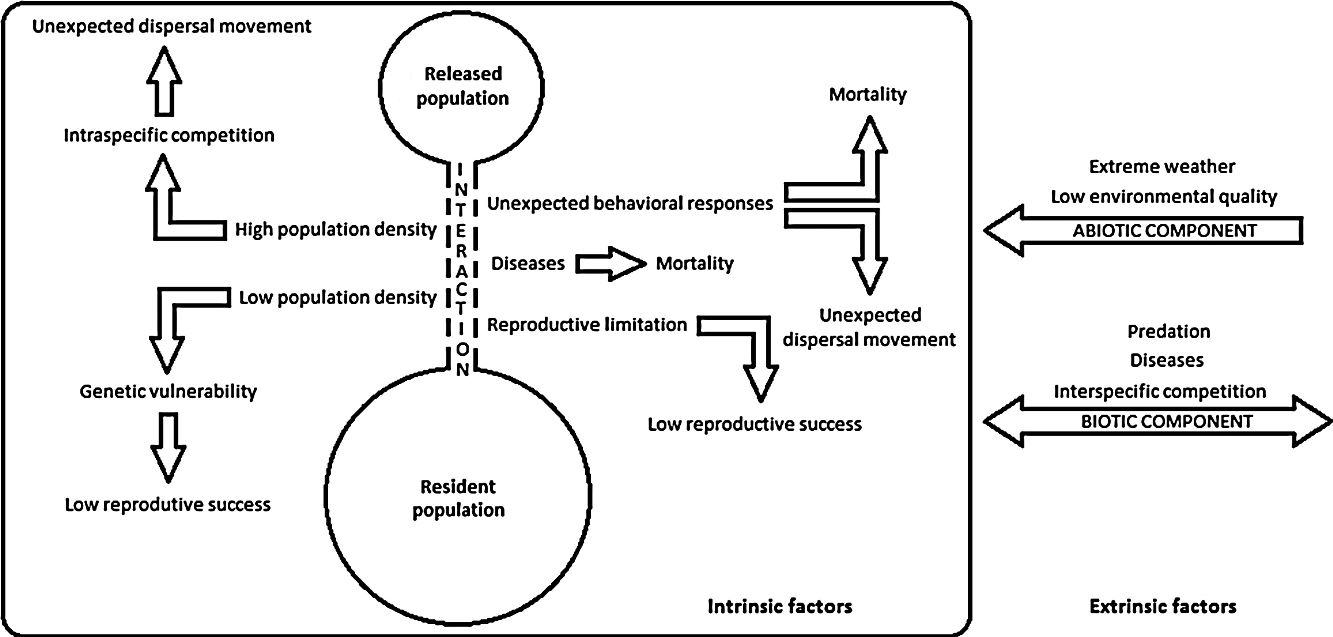
Despite recent efforts to develop the science of reintroduction biology, many issues are still the subject of inconclusive debate (White et al., 2012; Robert et al., 2015), and thus, pointing out reintroduction failures seems to be an easier and more viable alternative to evaluate reintroduction success (Robert et al., 2015). The environmental drivers that can negatively impact population restoration programs are listed through a conceptual model presented herein (see Supplementary Material – S.1). Intrinsic factors evidence interactions among reintroduced and resident populations, and extrinsic factors are related to other species or environment. Either isolated or taken together, these drivers may harm a reintroduced population by hampering its establishment, growth or regulation, or destabilize resident populations and ecological processes. Thus, in order to better understand these failure dynamics and be able to better plan prevention and control actions, we aimed herein to systematize the main drivers that can negatively impact the bird population restoration programs according to researchers and practitioners. In addition, the conservation status of the bird species and countries with the most attempts in population restoration were listed and evaluated.
MethodsOur search was performed on the online database ISI Web of Knowledge (www.isiknowledge.com) to identify papers published from 1990 to 2016 that report bird reintroduction or reinforcement attempts. Birds were chosen because, alongside mammals, this group presents the most available data (Champagnon et al., 2012; Seddon et al., 2014), probably due to their social image (Bajomi et al., 2010) or because they are relatively easily studied and rapid results can be obtained (Armstrong and Seddon, 2011). For the literature search, the terms “reintroduction” OR “reinforcement” AND “bird” OR “avian” were used. However, to fulfill the purposes of the study and better detail the presentation of the methods, experimental design and results, paper selection was restricted. Thus, the analysis conducted herein did not consider: (i) accidental translocations or other conservation translocation initiatives, such as Conservation Introduction (Assisted Colonisation or Ecological Replacement) (see IUCN, 2013); (ii) newsletter articles, published abstracts, books, book chapters, technical reports or other gray literature; (iii) strictly theoretical studies, such as population modeling; and, (iv) studies without direct results on reintroduction/reinforcement attempts or related to other fields of science in which these terms have another meaning (e.g. molecular biology).
In the final database, population restoration attempts were individualized according to species, country and year of release. Each species was featured according to its taxonomic family and conservation status (IUCN, 2017). Studies involving more than one species in a single article were individualized and considered as a unique restoration attempt (e.g. Miskelly et al., 2009), and identical restoration efforts presented in more than one article were grouped (e.g. Bernardo et al., 2011a,b). Altogether, 75 restoration efforts were identified which, although not resulting in an exhaustive bibliographical review, since researchers are more likely to report a “success” (Fischer and Lindenmayer, 2000), represent a reliable synthesis of peer-reviewed literature, less prone to bias and with quality assured information (Bajomi et al., 2010).
Each study was also categorized according to drivers that can negatively impact population restoration. These drivers were extracted from issues that researchers addressed in their research, reflecting their theoretical perspectives and problems they thought were relevant to the study. In sum, we identified: (i) environmental causes; (ii) anthropogenic causes; and (iii) unknown causes. Anthropogenic causes are those specifically related to failures during the pre and post-release management. Environmental causes are those who suffer the action of biotic components (e.g. predation, intra or interspecific competition or diseases), abiotic components (e.g. low environmental quality and extreme weather), or are the result of individual responses to release events or applied management (e.g. non-establishment of an animal in the release site, low population size, genetic vulnerability, reproductive limitation, nest abandonment or infanticide-chick cannibalism) (see Supplementary Material – S.1). The results were presented using tables and histograms that illustrate some of the most broad prevalent trends apparent in the data (Fischer and Lindenmayer, 2000). Thus, the most common species in this regard and their threat degree, the countries with the most restoration attempts and the main failures drivers were identified.
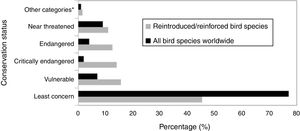
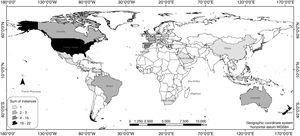
ResultsAccording to the review conducted herein, from 1990 to 2016, 64 bird species and subspecies across 33 different families were used in reintroduction/reinforcement attempts in 30 countries (see Supplementary Material – S.2). The most common species were Grus americana (5 instances) and Notiomystis cincta (3), and the most frequent families were Procellariidae (8) and Gruidae (5). Regarding conservation status, 45% of the species were classified as being of Least Concern, 16% as Vulnerable, 14% as Critically Endangered and 13% as Endangered (Fig. 1). The highest number of studies was carried out in New Zealand (22 instances), USA (16), Spain (4) and Japan (4) (Fig. 2).
IUCN Red List status for all the bird species worldwide (BirdLife International, 2017) and for all bird species compiled in this study. *Other categories: extinct, extinct in the wild and Data Deficient.
Thirteen drivers that may negatively impact reintroduction or reinforcement attempts were pointed out by researchers and practitioners in their studies (Table 1). Most studies presented two or more negative drivers, although some reports did not point out any obstacle. Considering only environmental causes, predation was the greatest impact (27 instances), followed by unexpected dispersal movement (24) and diseases (12). The most important anthropogenic causes were negative human interference (13 instances) (e.g. hunting, trampling or poisoning), pre-release management (12) (e.g. unexpected death during transport or inappropriate animal handling) and failures in post-release management (8) (e.g. lack of shelter or supplementary feeding). Unknown causes of mortality were identified in 18 studies.
Drivers reported as relevant by researchers and practitioners in bird population restoration programs. These were grouped as: (a) environmental; (b) anthropogenic; and (c) unknown.
In our study, predation was reported as the most common cause of failure for bird restoration (e.g. Pérez et al., 2004; Carrlson et al., 2014), in the same way that it seems to be the major problem in the population restoration of other animal groups (Short et al., 1991; Moseby et al., 2011). For birds, high predation threats significantly decrease overall success rates by reducing both post-release survival and the probability of subsequent breeding by released birds (White et al., 2012). Thus, in order to minimize predation impacts, some biologists suggest anti-predator behavioral training during the pre-release phase (White et al., 2005; Sanz and Grajal, 1998), or even predator removal by culling or translocation (Smith et al., 2010). Predator impact is even more worrying on islands, such as Hawaii (VanderWerf et al., 2014), or island countries, such as New Zealand (Miskelly et al., 2009), where the return of native species to the original range is only possible after the total eradication these predators/competitors (Leech et al., 2007; Richardson et al., 2013). For example, eight Procellariidae species, the family best represented in this study, could only be reintroduced to islands in New Zealand after the complete eradication of introduced mammals, such as cats (Felis catus) and rats (Rattus exulans) (Miskelly et al., 2009).
Unexpected dispersal movement of released individuals was another important failure driver highlighted herein. This driver decreases the possibility of the population establishment post-release (Dickens et al., 2009), mainly owing to either chance fates of those individuals (demographic stochasticity) and to low reproduction or survival rates of the remaining population, due to low densities – Allee effects (Caughley, 1994; Armstrong and Wittmer, 2011). In our study, we observed one critical reintroduction attempt involving Tuamotu kingfishers (Todiramphus gambieri gertrudae), where all animals returned to the donor area (Kesler et al., 2012). However, in contrast, another study with Mauritius kestrel (Falco punctatus) revealed that restricted dispersal affected territory occupancy patterns (Burgess et al., 2008). For Armstrong et al. (2013), post-release dispersal is a key factor affecting the success of ecological restoration projects, and therefore, the failure risk should be potentially reduced by managing dispersal, by translocating more animals to compensate for dispersal, or by avoiding release in areas prone to dispersal.
Pathologies, also highlighted herein, have always been a great concern for professionals involved in population restoration efforts, either due the transmitted diseases among released and resident populations or to interspecific forms (Candelora et al., 2010). Aspergillosis (Castro et al., 2004), poxviruses (Krone et al., 2004), and toxoplasmosis (Work et al., 2000) were some of the most commonly mentioned diseases. However, the majority of population restoration attempts around the world have applied intense veterinary protocols during the pre-release phase (e.g. Brightsmith et al., 2005; Bernardo et al., 2011a; Keller and Hartup, 2013), thus inhibiting disease transmission risks.
The Whooping Crane (Grus americana), an endangered North American migratory bird since 1967, was the most common species used in the restoration actions summarized herein. However, despite several reintroduction activities and conservation projects, the survival of this species is still worrying, mainly due to its low reproductive success, predation, and trauma caused by firearms or collisions (Cole et al., 2009; Converse et al., 2013). Overall, although the threat rate of the species analyzed herein was higher than the baseline rate of all known species from all threat categories (Fig. 1), most studies focused on bird species categorized as “Least Concern” (e.g. Leech et al., 2007; Dickens et al., 2009; Bennett et al., 2012). These data corroborate the study by Seddon et al. (2005), who observed that reintroduction project bias for both mammals and birds was not related to differences between orders regarding vulnerability to threat. In general, most bird species were released in areas where the original population still exists (reinforcement), usually aiming to evaluate different release methods through the surrogate species of the actual target species (Hardouin et al., 2014), or in sites where the target species had become locally extinct (e.g. Jamieson, 2011; Slater and Altman, 2011; Estrada, 2014). Moreover, in many tropical countries, such as Brazil, it is also common that trafficked animals return to capture areas after being seized by the authorities (Destro et al., 2012). Thus, the selection of candidates for reintroduction programmes does not only consider the threat degree of the species, but also national priorities, funding availability, and local community support over global conservation status (Seddon et al., 2005).
Regardless of environmental drivers that have been the main cause for reintroduction/reinforcement failures, some studies point out that direct or indirect human impact may be primarily responsible for the high mortality of released birds (Margalida et al., 2008; Rideout et al., 2012). Among the negative human impacts observed herein, the following are noteworthy: poisoning (Margalida et al., 2008; Rideout et al., 2012), hunting (Pérez et al., 2004), trauma and death caused by collision with vehicles or power infrastructures (Margalida et al., 2008; Mitchell et al., 2011; Keller and Hartup, 2013) and litter ingestion (Rideout et al., 2012). On the other hand, some species such Lesser Kestrels (Falco naumanni), one of the most endangered birds in Europe, may be favored by urbanization and human influence because they nest in crevices or cavities of farm buildings, old churches or castles (Pérez et al., 2011).
Although widely implemented worldwide, reintroduction or reinforcement actions carried out in island countries, such as New Zealand (Miskelly et al., 2009; Jamieson, 2011; Richardson et al., 2013) and other territories bordered by the sea, like Hawaii (Groombridge et al., 2004; VanderWerf et al., 2014), are again noteworthy, since they were the major focus of the studies analyzed herein. In these areas where natural resources are limited, certain environmental management measures are frequently performed before reintroduction efforts, such as habitat restoration and enrichment (Reynolds et al., 2008; Endo and Nagata, 2013), and control of exotic species (Groombridge et al., 2004). However, as they are considered dynamic processes modeled by intrinsic and extrinsic factors, release plans must be even more carefully planned out (Groombridge et al., 2004), requiring researchers and practitioners to recognize poor performance caused by internal weaknesses or other causes, so they can take remedial steps (Clark and Westrum, 1989). Furthermore, considering that only three of the 10 countries with the largest numbers of reintroduction/reinforcement efforts (Fig. 2) were listed among the 17 most megadiverse countries in the world (Mittermeier et al., 1997), with eight of these belonging to the list of the 15 largest economies (IMF, 2016), we can infer that population restoration efforts are concentrated in rich countries, instead of more megadiverse nations.
Population restoration is a long-standing practice (Jørgensen, 2013) and appears to be more successful when the source population is wild, when a large number of specimens is released, and when the cause of original decline was previously removed (Fischer and Lindenmayer, 2000). However, its success rate is often overestimated, since successful projects are more likely to be published than failed projects or those with uncertain outcomes (Miller et al., 2014). In fact, causes for restoration failures are difficult to evaluate due to lack of monitoring (Fischer and Lindenmayer, 2000), although an understanding of the frequency and causes of restoration failures is valuable for reintroduction biology and may be a singular way to understand the dynamics involved in population restoration actions (Miller et al., 2014). Herein, even though some species and restoration efforts have not been evaluated due to search criteria used, we have made an important contribution to the reintroduction biology, because we summarized the main failure drivers involved in bird population restoration attempts. Because of its generality, our review may also be applied in a wide variety of other studies, since, independent of the chosen bird species, many programmatic similarities among these animals and their conservation programs are noted (Clark and Westrum, 1989). Thus, we stimulate researchers and practitioners to predict these failure drivers during pre-release planning and, subsequently, evaluate them in the post-release monitoring stages, in order to better understand the actual problems inherent to population restoration programs.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
We thank the Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) for supporting our research, as well as anonymous reviewers for valuable suggestions on previous versions of this manuscript. LCT and PDMJ also thank the support provided by CNPq Productivity.