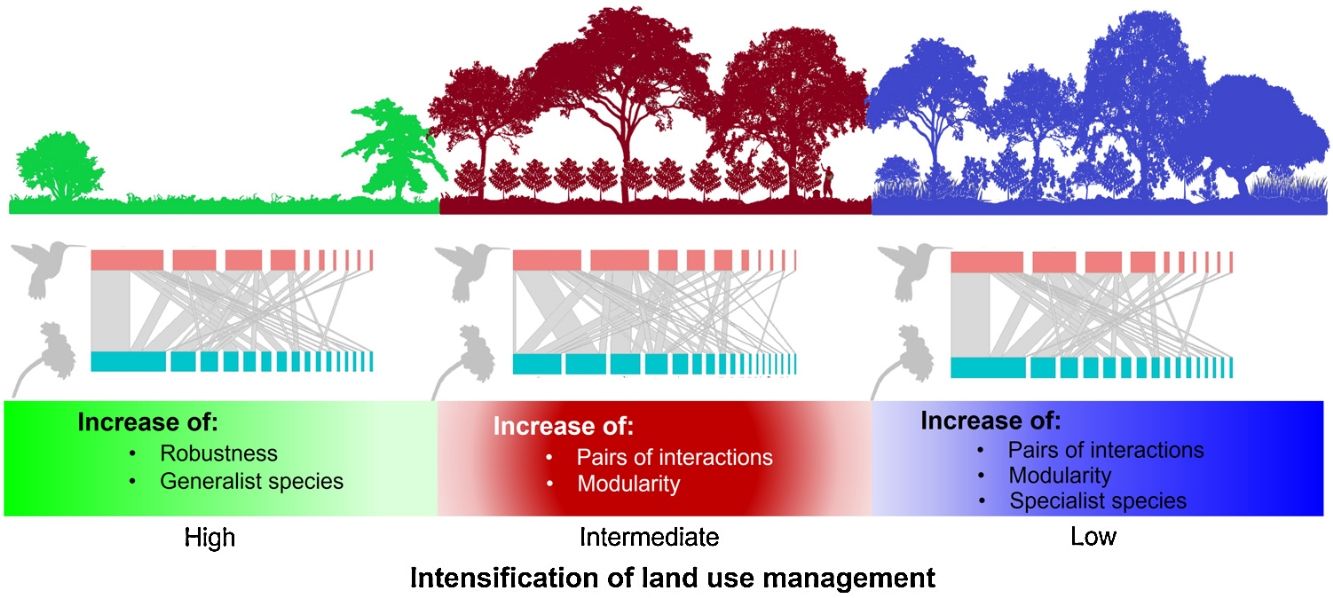
Agroforestry systems, such as shade coffee plantations, favor the maintenance of woody vegetation, which promotes the presence of pollinators such as hummingbirds. Many shade coffee plantations in Mesoamerica have been abandoned due to the fall in coffee prices and undergone succession processes that increase vegetation complexity. Alternatively, they have been replaced by cattle pastures, which negatively affect hummingbird-plant interactions. Here, we describe the structure of hummingbird-plant interaction networks in three types of land use―late-successional forests, coffee plantations, and cattle pastures―that were derived from a cloud forest in southern Mexico. For one year (2019–2020), we collected bimonthly quantitative data on hummingbird-plant interactions for each type of land use. We found that the network of each land use had a heterogeneous structure, and most species had few linkages. The late forests and coffee plantations had more species, pairs of interactions, and modularity than the cattle pastures. The cattle pasture network had the greatest robustness due to the presence of generalist hummingbirds, which are important for network cohesion in a great disturbance scenario. Furthermore, hummingbird visits were influenced by plant traits, such as foliage height diversity. The study findings suggest that the conversion of natural habitats have implications for the conservation of plant-pollinator interactions and that generalist pollinator species are key to disturbance resiliency.
Neotropical cloud forests (CFs) have the highest biodiversity per surface unit of all terrestrial ecosystems (Dirzo and Raven, 2003), and promote the development of multi-trophic relationships at different levels (Vizentin-Bugoni et al., 2018). However, they are one of the most threatened ecosystems worldwide (Hamilton et al., 1995; Scatena et al., 2010). Specifically, in the neotropics more than 41% of CFs have been converted into secondary forests, agricultural land, or pastures (Martínez et al., 2009; Gibbs et al., 2010; Mendoza-Ponce et al., 2018). These anthropogenic changes negatively affect the composition, structure, and ecological functions of biotic communities (Şekercioğlu et al., 2004; García et al., 2011; Sandor et al., 2022). Despite the incessant increase in agricultural and livestock-related activities in converted CF areas, some crops decrease susceptibility of ecological functions (Harvey et al., 2021). For example, rustic shade coffee plantations maintain the essential ecosystem services provided by biodiversity through multiple interactions between plants and animals (Martínez-Salinas et al., 2022).
Coffee cultivation represents one of the most productive activities in the humid tropics of the Americas, with around 5.2 million hectares cultivated (FAO, 2016), which are often located within forest areas in peasant communities and generally support great biodiversity (Moguel and Toledo, 1999). A large extension of these plantations tends to grow under the canopy of humid mountain forests and have been considered as “friendly” to biodiversity conservation and a great provider of ecosystem services (Williams-Guillen and Perfecto, 2011; Alvarez-Alvarez et al., 2021; Harvey et al., 2021).
Several studies have shown that in these plantations, different bird groups find refuge under various disturbance scenarios and spatial scales (Greenberg et al., 1997; Smith et al., 2015). Other studies have shown that these plantations maintain the structure and composition of the original tree vegetation and provide bird groups with the resources they need to survive (e.g., Tejeda-Cruz and Sutherland, 2004). In addition, it has been demonstrated that some bird groups provide essential services by controlling pests, such as the coffee borer (Hypothenemus hampei), which causes extensive damage to coffee plantations worldwide (Damon, 2000). To a lesser extent, birds can also contribute to the pollination of this crop (Whelan et al., 2015).
In Latin America, many shade coffee plantations have been abandoned due to falling coffee prices (Canet and Soto, 2016; Harvey et al., 2021) or replaced by activities that intensify land use, such as cattle ranching (Alvarez-Alvarez et al., 2022). At abandoned coffee plantations, shrubby vegetation and trees typically undergo regeneration and/or succession processes, which makes the vegetation more complex (Alvarez-Alvarez et al., 2021). Both in highly disturbed areas, such as cattle ranching, or in current or abandoned coffee plantations, changes in landscape composition promote changes in plant and bird species composition, disrupting their interactions (García et al., 2011; Morrison and Mendenhall, 2020). However, studies of bird-plant interactions in sites with different agroforestry management regimes have not been addressed in tropical landscapes. In particular, the interactions of bird groups that perform important ecological functions, such as pollination, have not been sufficiently studied.
Mutualistic interactions between pollinators and plants are among the most important interactions in natural systems, as approximately 70–90% of flowering plants are pollinated by animals (Bascompte and Jordano, 2007; Ollerton et al., 2011). In the Americas, hummingbirds are the most important birds that carry out this ecological function. Buzato et al. (2000) estimated that hummingbirds pollinate 10–15% of angiosperms throughout the neotropics. Moreover, both hummingbirds and plants have diversified due to their interactions and the existence of high levels of reciprocal specialization (Serrano-Serrano et al., 2017).
Pollinator visitation is determined by different traits of both plants and pollinators. In this context, bill size and morphology are one of the most important traits of hummingbirds (Maglianesi et al., 2014; Izquierdo-Palma et al., 2021), while nectar characteristics and the number, size, shape, and color of flowers are important plant-based factors (Fornoff et al., 2017). Meanwhile, other variables that have been recognized as good predictors of bird diversity, such as foliage height diversity (MacArthur and MacArthur, 1961), have rarely been considered in studies of interactions between plants and their floral visitors (e.g., Klecka et al., 2018). The foliage height diversity supposes that greater vertical stratification provides avian communities with resources and microhabitats that serve as feeding, refuge, and reproduction sites (Chmel et al., 2016; Almazán-Núñez et al., 2021). Knowledge of interaction networks and the variables that drive these mutualisms may be relevant to a better understanding of tropical agroforestry ecosystems.
Studies of heavily modified landscapes have found that some hummingbird species are resistant to disturbance (Sonne et al., 2016; Bustamante-Castillo et al., 2018; Maruyama et al., 2019), and may establish new interactions with plants, particularly invasive ones (Aizen et al., 2008), resulting in less functionally diverse communities and more generalist networks (Maruyama et al., 2019). Studies on interaction networks in modified primary forests have revealed the flexibility and vulnerability of mutualisms in the face of species loss (Bascompte et al., 2003, 2006; Morrison and Mendenhall, 2020). Thus, ecological networks serve as a valuable tool within applied ecology because they can be used to monitor the impact of anthropogenic disturbances on ecosystems and evaluate the efficiency of restoration programs (Kaiser-Bunbury and Blüthgen, 2015).
In this study, we describe the composition of hummingbird-plant interaction networks in three types of land use: (1) forests in advanced succession (hereafter, late forest), (2) shade coffee plantations (hereafter, coffee plantations), and (3) cattle pastures, that had been derived from CF in southern Mexico. We also test whether there is an association between plant structural traits and the number of hummingbird visits to flowers in relation to types of land use. Based on these aims, we propose the following predictions:
- 1.
Hummingbird-plant interactions will be more intense and connected in late forests and coffee plantations compared to cattle pastures, due to their diverse floristic composition and complex vegetation structure.
- 2.
Cattle pasture networks will have greater nestedness and generalization than late forests because habitat specialists tend to be affected by disturbed environments (Beal-Neves et al., 2020).
- 3.
Cattle pastures will display a more robust network, favored by the presence of invasive plant species which are more likely to create new connections with generalist hummingbirds (Aizen et al., 2008; Díaz-Infante et al., 2020).
- 4.
Interactions along the disturbance gradient will be influenced by the foliage height diversity because stratification enables the coexistence of bird species that exploit similar resources (Chmel et al., 2016).
The study findings have important implications for the conservation of mutualistic pollination interactions in CFs ecosystems, which are one of the most threatened ecosystems worldwide.
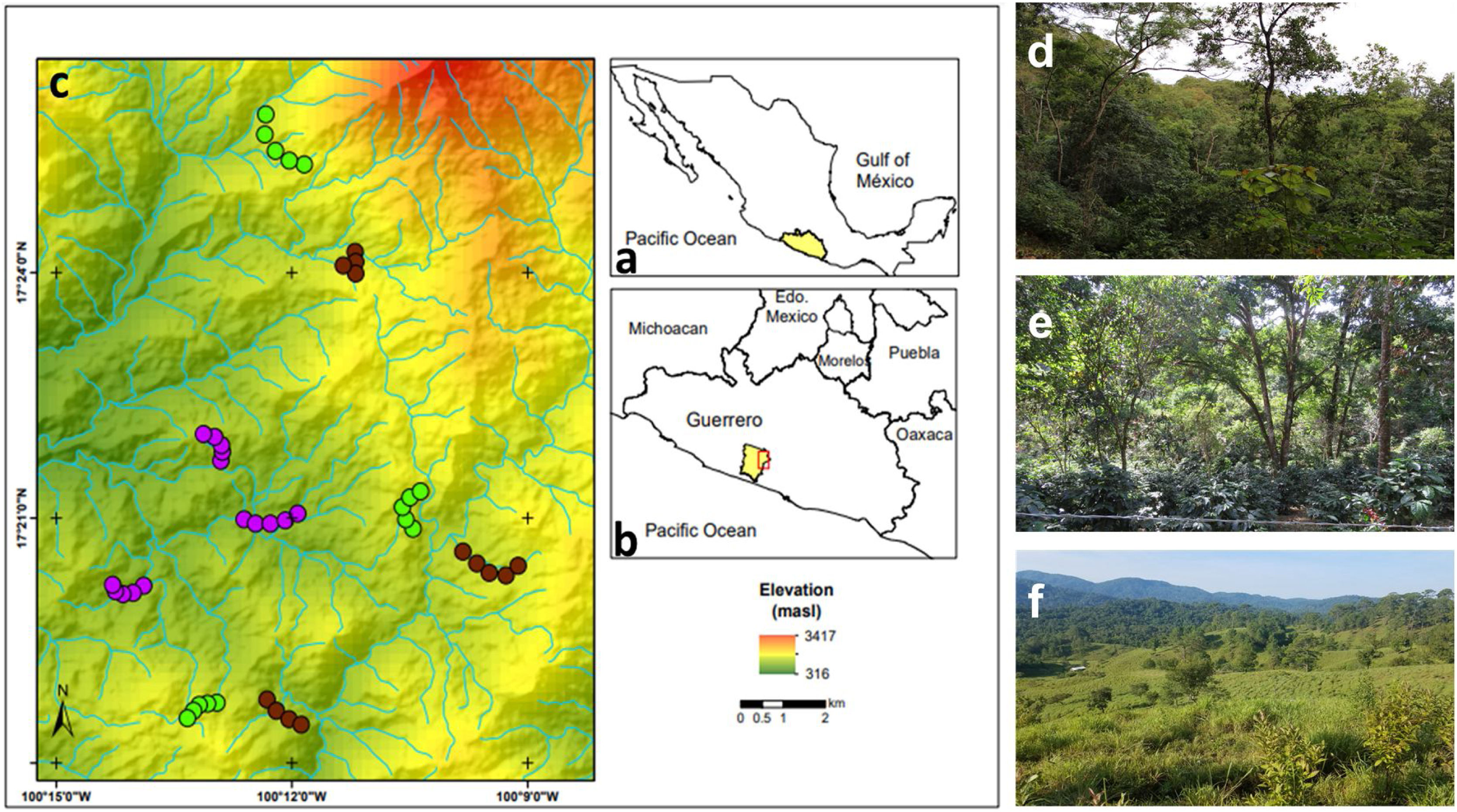
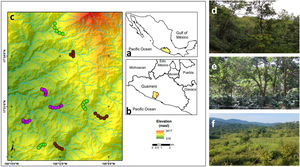
MethodsStudy area and sampling sites descriptionThe study area is in the Sierra de Atoyac, which is located in the Sierra Madre del Sur biogeographic province in the state of Guerrero, southern Mexico (Fig. 1a, b). The area has a temperate sub-humid climate with an average annual temperature of 20–24 °C and 1500–2000 mm of rainfall (INEGI, 2010). The main vegetation type is CF with different land use regimes but predominantly associated with shade coffee plantations. Tropical sub-deciduous and pine-oak forests are also present. Three types of land use, derived from the CF, were selected: (1) cattle pastures, (2) coffee plantations, and (3) late forests (Fig. 1c). Cattle pastures are open areas with sparse vegetation, used mainly for cattle grazing with slopes of 15° to 20° (Fig. 1d). In these sites, it is common to find an herbaceous and shrubby layer, while a tree layer is uncommon and usually used as living fences. Coffee plantations are sites where the herbaceous and shrubby layer was removed for coffee crop (Coffea arabica), but maintain arboreal elements of the original vegetation (Fig. 1e). Finally, late forests are characterized by a dense canopy that was used for coffee plantations approximately 35 years ago (Fig. 1f); yet these are currently abandoned orchards, and succession processes have allowed the regeneration of shrubby and arboreal species (e.g., Inga vera and Clethra fragans, Alvarez-Alvarez et al., 2021). Both late forests and coffee plantations have a rugged orography with slopes of 30° to 45°. For each type of land use, three replicates were selected (i.e., nine sites in total). At each site, a 2-km-long transect was established, where the only criterion considered for inclusion was that they had the presence of floral patches, although they were not necessarily species with the ornithophily syndrome. The minimum distance between the sites of the three land uses was 1 km.
(a) Geographic location of the state of Guerrero in southern Mexico, (b) the study area in the municipality of Atoyac de Álvarez, and (c) the observation transects belonging to each type of land use. Overview of the (d) late forests, (e) coffee plantations, and (f) cattle pastures in the study area. Purple circles represent cattle pastures, brown circles represent coffee plantations, and green circles represent late forest sites.
In each transect, patches with a high concentration of flowers were located, and different plant growth forms (i.e., herb, shrub, tree, vine, and epiphyte) were considered. The interactions between hummingbird and plant species were recorded via direct observations that were carried out bimonthly from March 2019 to May 2020. Binoculars and specialized guides were used to identify hummingbird species (e.g., Howell, 2002; Arizmendi and Berlanga, 2014). Four visits were made to each site of three land uses (12 visits per land use). The observation time coincided with the peak hours of bird activity (07:00 to 10:00 and 16:00 to 19:00). Each flower patch was observed for 10–15 min, and the observation time for each plant species in the floral patches was proportional to its abundance in the transects. An average of three observation hours was invested in each transect (i.e., ∼12 h per site, and ∼36 h per land use). We identified the plants with which the hummingbirds interacted and determined the frequency of the visits by measuring the number of times a hummingbird fed on at least one flower per plant species (Maruyama et al., 2019).
Plant species that were observed in interactions with hummingbirds but not identified in situ were collected and photographed for later determination. The collected specimens were deposited in the herbarium of the Facultad de Ciencias Químico Biológicas of the Universidad Autónoma de Guerrero. Floral abundance was estimated by counting the number of open flowers or inflorescences per plant species and per transect in a 10-m-wide strip (Rodrigues and Rodrigues, 2015). Data on foliar cover (a horizontal measure defined as the proportion of the ground occupied by the vertical projection of foliage; Walker and Hopkins, 1990) and foliage height diversity (a vertical measure indicating foliar strata diversity; MacArthur and MacArthur, 1961), were collected for each type of land use (Alvarez-Alvarez et al., 2021). To measure foliar cover and foliage height diversity, in the same transects in which the interaction observations were made, five plots per site were randomly delimited (15 plots per land use type, 45 plots in total). Each plot had an area of 0.28 ha and the distance between plots was 200 m.
To characterize the structure of the woody vegetation, two cross-sectional transects, in which two perpendicular lines were oriented to the four cardinal points and demarcated with a rope, were established in each plot at each site. Foliage cover was estimated for each tree and shrub species using an ellipse formula based on the maximum and minimum diameters (Muller-Dombois and Ellenberg, 1974). Foliage stratification was estimated using an optical square marked with two perpendicular axes (Montaña and Ezcurra, 1980). Three mirrors were arranged in the square so that the height of the objects could be determined looking horizontally through the device. In each plot, foliage height and the number of times that the foliage touched the point of intersection of the two axes were recorded (Almazán-Núñez et al., 2016). This procedure was repeated every 1-m along the two transects from the center to the four cardinal points. The recorded heights were grouped into 2-m class intervals, and foliage height diversity was assessed using the Shannon-Wiener index.
Network structure and metricsTo analyze the structure of the hummingbird-plant interactions, qualitative and quantitative interaction matrices were constructed for each type of land use (combining data from the three replicates by land use). In the matrices, the columns represented the hummingbird species, while the rows represented the plant species (Maruyama et al., 2018, 2019). A value of 1 in the cells in the qualitative matrix indicated that there was an interaction between the network partners, while a value of 0 indicated that there was no interaction (Dormann et al., 2008). In the quantitative matrix, the value of the cells corresponded to the total number of visits of the hummingbird species to the plant species (Dormann et al., 2008; Maruyama et al., 2018).
Network-level metricsFor the qualitative matrix, we estimated the cumulative frequency distribution of the number of interactions per node and tested the fit to the following distribution types: (1) exponential, (2) power law, and (3) truncated power law (Jordano et al., 2003; Bascompte and Jordano, 2007). In addition, we determined which distribution type best fit the original data distribution based on the lowest Akaike Information Criterion (AIC) value. For mutualistic networks, a preferential attachment is expected, in which each node has a maximum number of potential links (Jordano et al., 2003). We used an analysis of degree distribution to explore the heterogeneity of the interaction distributions among the nodes (independent of interaction intensity) instead of just quantifying the degree of each node (based on the number of links per node). Furthermore, the analysis of degree distribution allowed us to detect this “truncation” in the matrices for each type of land use, supporting the fact that hummingbird species preferentially bind to the plants they use as nectar sources, regardless of the type of land use.
We calculated nestedness, which is a metric that quantifies the degree to which the interactions between specialist species are subsets of the interactions between more generalist species in the network (Jordano, 2010). This metric was calculated for each land use matrix using NODF and wNODF parameters (Almeida-Neto et al., 2008; Almeida-Neto and Ulrich, 2011).
We also calculated the network modularity, which is defined as the presence of subgroups or modules of species that tend to interact more strongly among themselves than with other modules (Bascompte and Jordano, 2007; Jordano, 2010). This metric was calculated for each network by land use using the QuanBiMo (Q) algorithm, which was developed for weighted bipartite networks (Thébault, 2013). The Q algorithm values were used to classify the hummingbird and plant species in the network based on roles proposed by Olesen et al. (2007) using two criteria: the value z, which measures the degree of connection of a species within a module, and the value c, which quantifies these connections in all modules (Dáttilo et al., 2016). Criteria z and c have threshold cutoff values to separate the species role within and between modules at the 95% percentile (from the lowest to the highest values based on the mean) and allow species to be ranked as module hubs (with several interactions within its module), network hubs (which give coherence to the network and their own module), connectors (which link different modules), and peripherals (which have few interactions with other species; Olesen et al., 2007; Dáttilo et al., 2016). The limit values of z and c were estimated from the average modularity of 100 null matrices generated by the FF model. To objectively define these thresholds, it was necessary to run null models of the original network and employ 95% quantiles as critical c- and z-values (Dormann and Strauss, 2014).
We also measured the specialization of each type of land use at the network level (H2′). This metric is a measure of the niche segregation between species and describes the extent to which observed interactions deviate from those expected based on the total number of interactions (Blüthgen et al., 2006).
Species-level metricsWe calculated specialization at the species level (d'). Derived from the Kulback-Leibler distance (as well as the Shannon–Wiener diversity index), this metric indicates how strongly a species deviates from a random sample of available interacting pairs (Blüthgen et al., 2006). Thus, it compares the interaction distribution of each partner to the general availability of the partners in the network.
We calculated robustness to describe the tolerance of hummingbird and plant networks to the extinction of their component species as a result of land use changes. This metric measures a system's tolerance to species loss through the area under the extinction curve (second.extinct; Memmott et al., 2004) based on the likelihood that the elimination of a given fraction of the species in one guild will lead to the extinction of a number of species in another guild based on their interactions. A system with low robustness has values close to 0, while system with high robustness has values close to 1 (Burgos et al., 2007).
We also performed removal scenarios for each type of land use. In the first simulation, we randomly removed nodes (hummingbirds and plants). In the second simulation, we eliminated hummingbird and/or plant species in a sequence from the most to the least connected. In the third simulation, we defined an extinction criterion according to the threat degree of the nodes. To do this, we eliminated hummingbirds first, especially species with a small range, according to BirdLife (2020); these species are also in some risk categories, according to the International Union for Conservation of Nature (IUCN, 2020; e.g., Lophornis barchylophus, Eupherusa poliocerca; Table S1). Regarding the plant removal sequence, we removed native, endemic, and IUCN-listed species first (i.e., Clethra fragrans, Inga eriocarpa, Table S2). Finally, we established a center-periphery structure for each network to enable the identification of species that were densely (Gc > 1, generalist core) or sparsely (Gc < 1, peripheral nodes) connected to other species of the same trophic level (Dáttilo et al., 2013).
Data analysisTo estimate the significance of the observed NODF nestedness value, 1000 null matrices were constructed using two algorithms: 1) vaznull function (bipartite package; a model with constrained connectance and moderately constrained marginal totals), and 2) swap function (metacom package; the most conservative null model with constrained connectance and marginal totals). Nestedness was estimated for each null matrix, and the z-score was calculated between the null models and the observed value, and values greater than the absolute value of two were considered statistically significant. For wNODF nestedness, modularity, specialization, and robustness of the network, the same protocol was followed using two types of null models: Patefield (r2dtable command in bipartite package; an intermediate model in which the marginal totals are constrained in the randomizations) and FF (swap_count command in metacom package; the stricter null model that maintains the same connectance, marginal totals, and the proportion of unrealized interactions; Dormann et al., 2008).
A generalized linear mixed model (GLMM) with Poisson-type error distribution was fitted to assess the effect of the total number of flowers, foliar cover, and foliage height diversity on the number of hummingbird visits for each type of land use. The total number of flowers, foliar cover, and foliage height diversity were included in the model as fixed factors, while the nine sampling sites and three types of land use were included as random factors.
The analyses associated with the bipartite networks were performed using the brainwaver packages for the frequency distribution, and bipartite, vegan, and metacom for the network metrics (Dormann et al., 2008; Olesen et al., 2008; Dallas, 2014; Oksanen et al., 2019). GLMM was carried out using the glmer function of the lme4 package (version 1.1-27.1; Bates et al., 2019). All analyses were performed in R 3.4.2 (R Development Core Team, 2017).
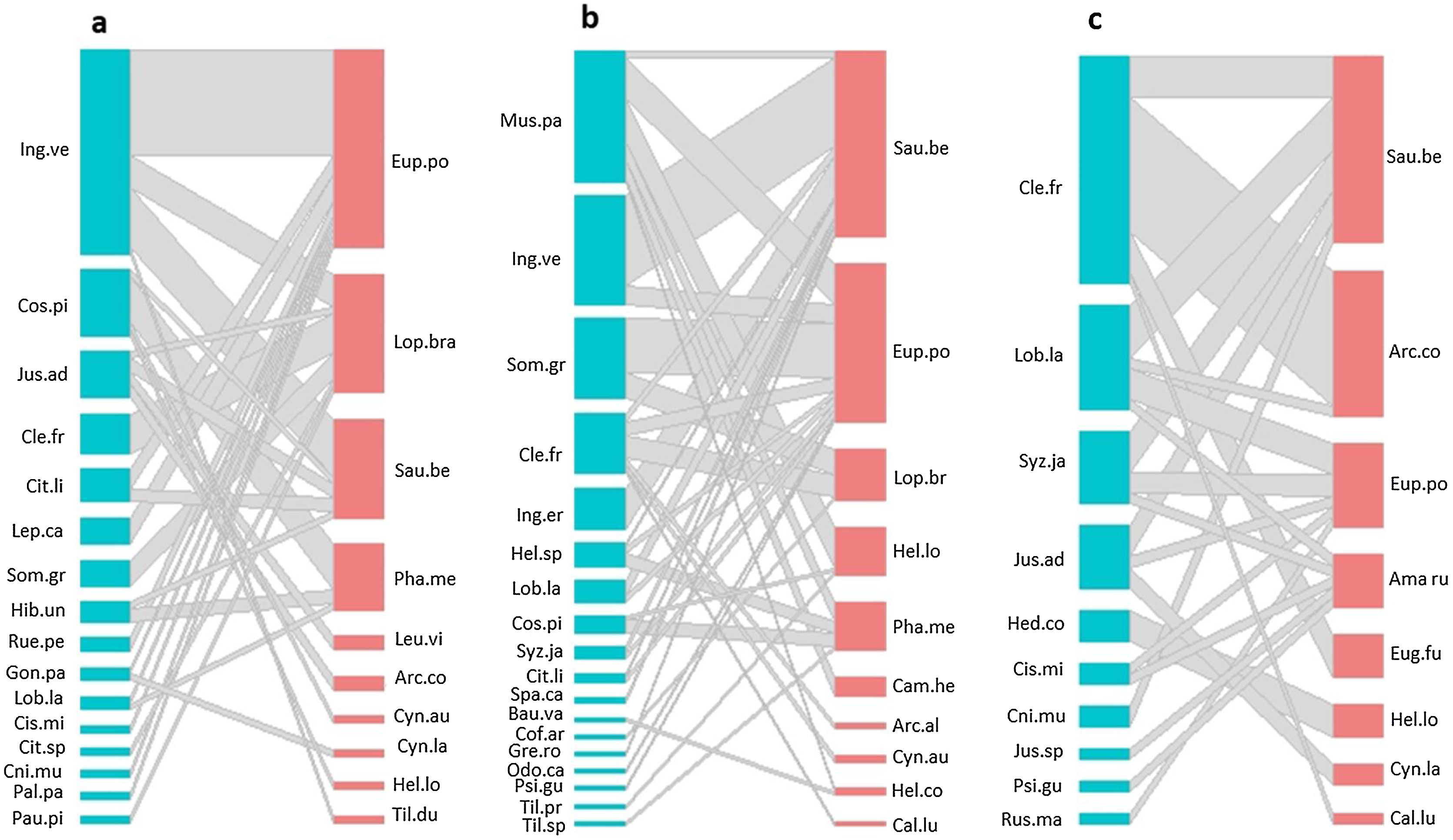
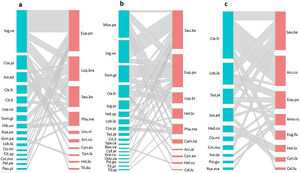
ResultsHummingbird-plant interaction networks between different types of land useWe recorded 31 plant species that were visited by 16 hummingbird species (Table S3). The hummingbird and plant species richness were greatest in the late forest and the coffee plantations. Both types of land use had 10 hummingbird species, and there were 16 plant species in the late forest and 18 in the coffee plantations (Fig. 2a–c). In the cattle pastures, there were eight hummingbird species and ten plant species. The highest number of interactions between plant species and hummingbirds was recorded in coffee plantations with 36 links, followed by late forests with 29, and cattle pastures with 21 (Fig. 2a–c). Some hummingbird species (e.g., Saucerottia beryllina and E. poliocerca) and plant species (e.g., Clethra fragans and Lobelia laxiflora) were common to all types of land uses. Other species were unique to certain types of land use. For example, the Gonzalagunia panamensis and Palicourea padiflora plant species and the Tilmatura dupontii hummingbird species only interacted in the late forests. The Heliomaster constantii, Campylopterus hemileucurus, and Archilochus alexandri hummingbird species and the Odontonema callistachyum, Spathodea campanulata, Musa paradisiaca, and C. arabica plant species only interacted in the coffee plantations. The Amazilia rutila and Eugenes fulgens hummingbird species and the Justicia spicigera, Russelia maculosa, and Hedychium coronarium plant species only interacted in the cattle pastures (Fig. 2a–c, Table S3).
Quantitative networks of hummingbird-plant interaction in three land uses derived from a CF in southern Mexico: a) late forest, b) coffee plantation, and c) cattle pasture. Blue blocks represent plant species, and red blocks represent hummingbird species. The block size is proportional to the number of interactions of each species. Plant and hummingbird name codes can be found in Table S3.
The connectivity of the network by type of land use was heterogeneous (Fig. 2a–c). The most connected nodes in all networks (center-periphery analysis) were the S. beryllina and E. poliocerca hummingbird species and the Inga vera, C. fragans, M. paradisiaca, and Sommera grandis plant species (Fig. 2a–c, Table S3). The heterogeneity in the number of links by node was supported by the observed frequency distribution of the matrices for each type of land use, which was fitted to a power law distribution as expected for mutualistic interactions (Fig. S1, Table S4).
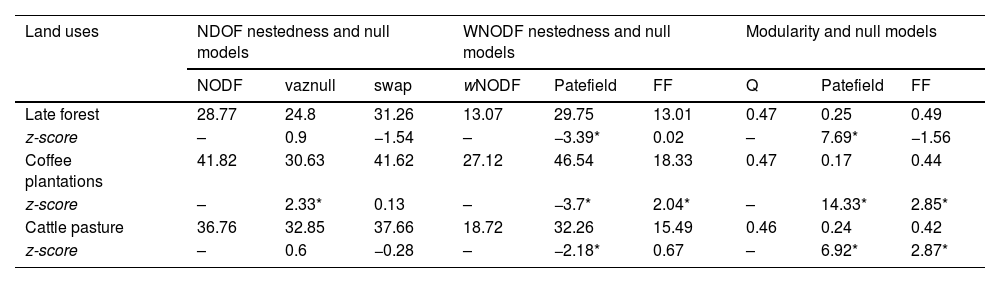
The significance of the NODF and wNODF values changed between the null models (Table 1). The three quantitative networks were significantly less nested than the null networks, according to the intermediate model (i.e., Patefield; Table 1). Regarding the qualitative matrices, nestedness was not significant for the late forest and cattle pastures. In the coffee plantations, the NODF value was significant and higher than the values in the randomized networks (Table 1). The modularity estimators indicated that the networks of each type of land use had compartmentalized structure and were significantly more modular than the null networks, according to the Patefield model (Table 1, Fig. S2).
Nestedness and modularity values for qualitative and quantitative interaction networks between hummingbirds and plants in three types of land use derived from a CF in southern Mexico. Null model values for each data type are displayed, and z-score values lower than -2 and higher than 2 denote significant differences against the null models (*denotes significance at P < 0.05).
| Land uses | NDOF nestedness and null models | WNODF nestedness and null models | Modularity and null models | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NODF | vaznull | swap | wNODF | Patefield | FF | Q | Patefield | FF | |
| Late forest | 28.77 | 24.8 | 31.26 | 13.07 | 29.75 | 13.01 | 0.47 | 0.25 | 0.49 |
| z-score | – | 0.9 | −1.54 | – | −3.39* | 0.02 | – | 7.69* | −1.56 |
| Coffee plantations | 41.82 | 30.63 | 41.62 | 27.12 | 46.54 | 18.33 | 0.47 | 0.17 | 0.44 |
| z-score | – | 2.33* | 0.13 | – | −3.7* | 2.04* | – | 14.33* | 2.85* |
| Cattle pasture | 36.76 | 32.85 | 37.66 | 18.72 | 32.26 | 15.49 | 0.46 | 0.24 | 0.42 |
| z-score | – | 0.6 | −0.28 | – | −2.18* | 0.67 | – | 6.92* | 2.87* |
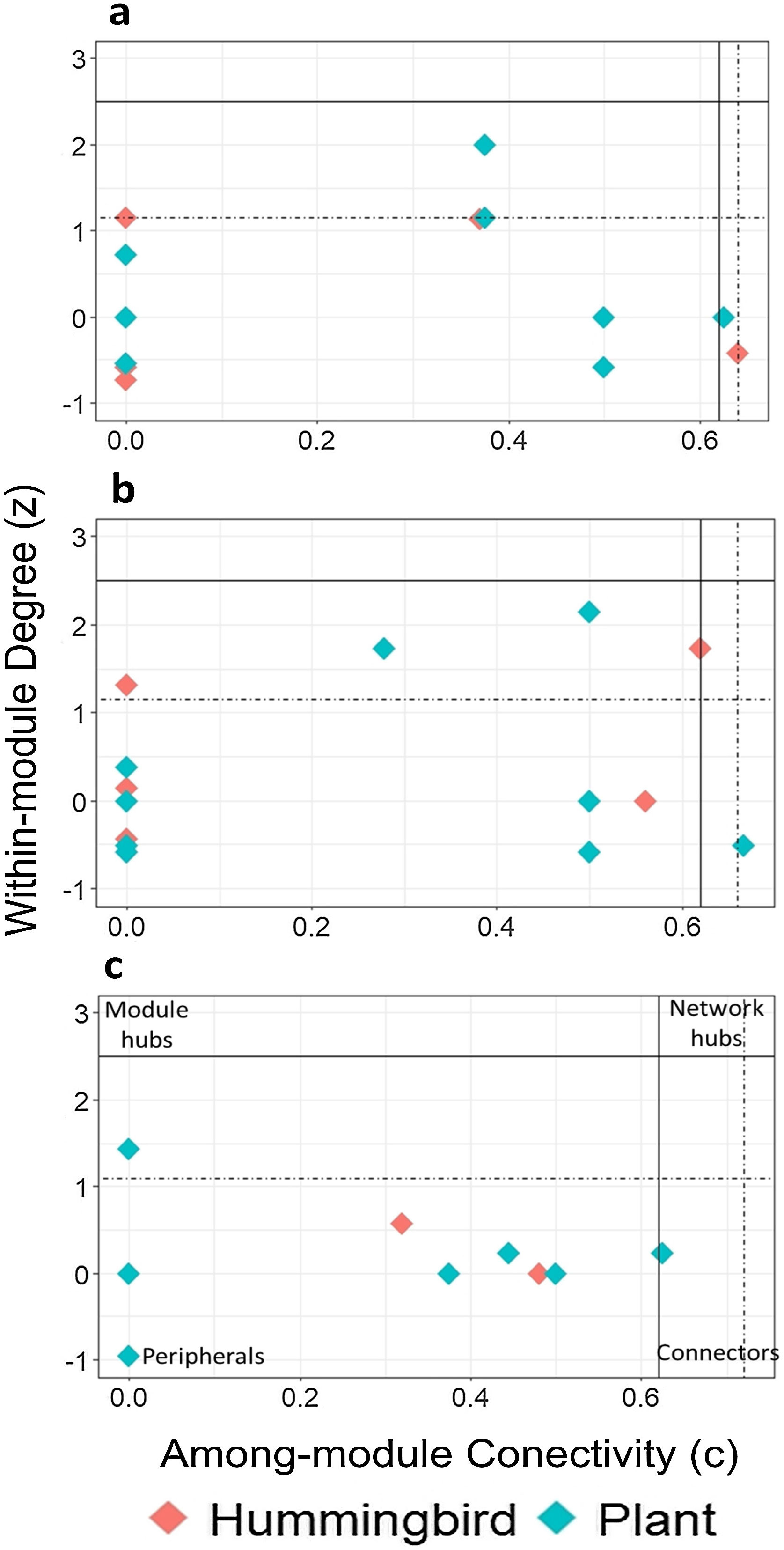
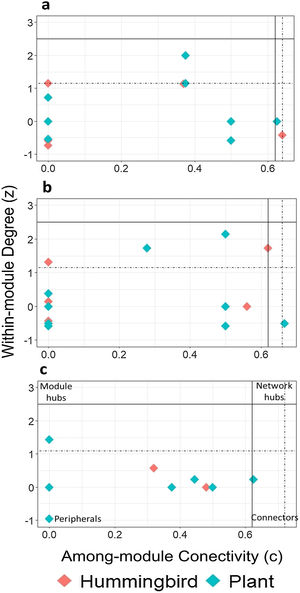
Using the estimated boundary values of the FF model (Table S5), the nodes in the late forest network, in addition to peripheral and connecting nodes (S. beryllina and Justicia adenothyrsa), three nodes were module hubs (the Phaethornis mexicanus hummingbird species and the I. vera and Costus pictus plant species; Fig. 3a). In the coffee plantation network, most nodes were peripheral, Heliconia spp. was a connector, and four nodes were module hubs (Fig. 3b). In the cattle pasture network, J. adenothyrsa was a module hub and the rest of the nodes were peripheral (Fig. 3c).
Classification of hummingbirds (red dots) and plants (blue dots), in accordance with modularity roles: a) late forest, b) coffee plantation, and c) cattle pasture network in southern Mexico. The y-axis represents the values of z (a measure of the number of connections of a species within its own module) and the x-axis represents the value of c (a measure of the connectance of a species with other species within other modules). The solid lines correspond to the cut-off values according to Olesen et al. (2007): z = 2.5 (horizontal line) and c = 0.62 (vertical line). The dotted lines represent the limit values obtained from the null matrices, using the FF model.
The specialization values (H2′) of the network by land use were higher than expected by chance (Table S6). The late forest network had five specialist hummingbird species (d′), including L. brachylophus, an endemic, endangered species. The coffee plantation and cattle pasture networks had three and two specialist hummingbird species, respectively. The cattle pastures network had the largest number of generalist hummingbird species (Table S6). Meanwhile, there were nine, seven, and three generalist plant species in the late forests, coffee plantations, and cattle pastures, respectively (Table S6).
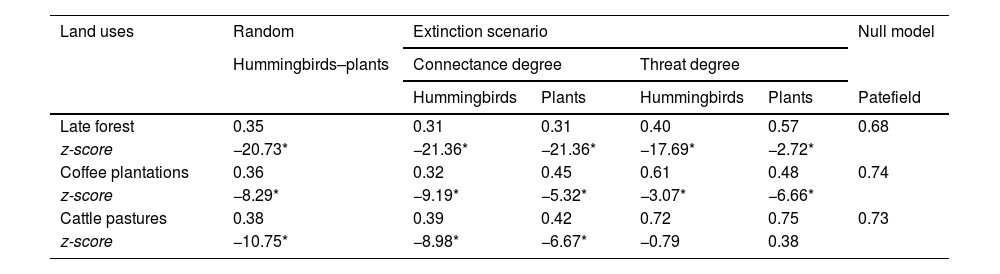
The network of each type of land use had low robustness (Table 2). When species were removed based on the connectance degree, the networks were more resistant to plant species loss than hummingbird species loss. When nodes were eliminated based on their threat degree, the late forest and coffee plantation networks were less robust than expected by the null model; only the cattle pasture network had greater robustness with the elimination of plants by threat degree, with respect to the null model (Table 2).
Robustness values under three extinction scenarios of the three types of land use networks derived from a CF in southern Mexico. z-score values lower than -2 and higher than 2 denote significant differences against the null models (*denotes significance at P < 0.05).
| Land uses | Random | Extinction scenario | Null model | |||
|---|---|---|---|---|---|---|
| Hummingbirds–plants | Connectance degree | Threat degree | ||||
| Hummingbirds | Plants | Hummingbirds | Plants | Patefield | ||
| Late forest | 0.35 | 0.31 | 0.31 | 0.40 | 0.57 | 0.68 |
| z-score | −20.73* | −21.36* | −21.36* | −17.69* | −2.72* | |
| Coffee plantations | 0.36 | 0.32 | 0.45 | 0.61 | 0.48 | 0.74 |
| z-score | −8.29* | −9.19* | −5.32* | −3.07* | −6.66* | |
| Cattle pastures | 0.38 | 0.39 | 0.42 | 0.72 | 0.75 | 0.73 |
| z-score | −10.75* | −8.98* | −6.67* | −0.79 | 0.38 | |
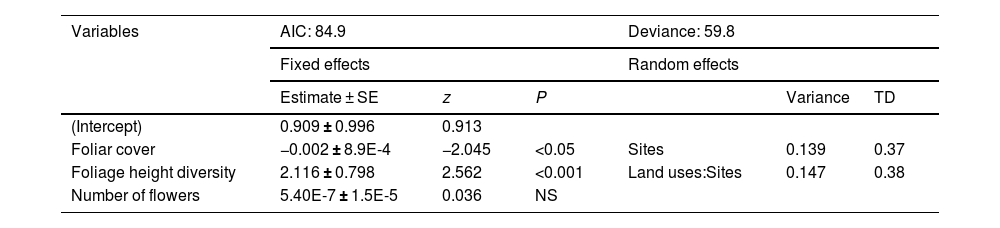
Foliar cover values and foliage height diversity were consistently higher in the late forests and coffee plantations compared to cattle pastures (Table S7). The late forest had the lowest number of flowers. The GLMM results showed that foliage height diversity had a very strong positive effect on the number of hummingbird visits to plants, while foliar cover had a negative effect (Table 3). Although the number of flowers had a positive effect on the frequency of hummingbird visits to the plants, it was not significant (Table 3).
Mixed generalized linear model to evaluate the effect of variables on the number of hummingbird visits to plants in three types of land use, derived from a CF in southern Mexico. SE: standard error, TD: typical deviation, NS: non-significant.
| Variables | AIC: 84.9 | Deviance: 59.8 | ||||
|---|---|---|---|---|---|---|
| Fixed effects | Random effects | |||||
| Estimate ± SE | z | P | Variance | TD | ||
| (Intercept) | 0.909 ± 0.996 | 0.913 | ||||
| Foliar cover | −0.002 ± 8.9E-4 | −2.045 | <0.05 | Sites | 0.139 | 0.37 |
| Foliage height diversity | 2.116 ± 0.798 | 2.562 | <0.001 | Land uses:Sites | 0.147 | 0.38 |
| Number of flowers | 5.40E-7 ± 1.5E-5 | 0.036 | NS | |||
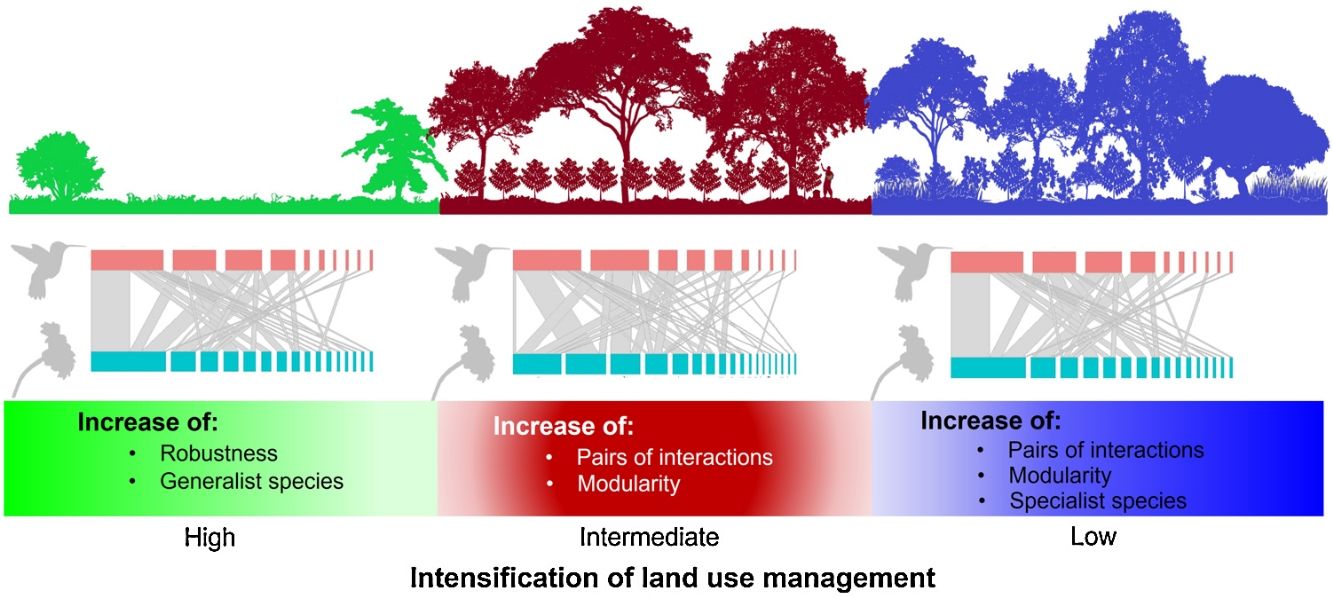
Our results showed how the composition of hummingbird and plant species is affected as land use change intensifies in a predominantly agroforestry landscape and the direct effect of such change on the structure and configuration of interaction networks. According to our first prediction, the coffee plantations and late forests had more plant and hummingbird species and interactions that the cattle pastures. Given that shade coffee plantations generally maintain tree cover (Valencia et al., 2014), which is a stratum that does not usually differ from that found in late forests (Alvarez-Alvarez et al., 2021), these agroforestry systems allow some pollinators and canopy species to increase their presence in these plantations (Vergara and Badano, 2009). Additionally, coffee plantations are usually combined with banana plantations (M. paradisiaca), which are frequently visited by some hummingbird species (Itino et al., 1991). Conversely, in cattle pastures, sparse vegetation cover is the main factor in the decreased food supplies of some pollinators; consequently, the number of floral visitors and their interactions are significantly affected, as has been reported in other studies (e.g., Bustamante-Castillo et al., 2018; Maruyama et al., 2019).
The networks of the three types of land use had a heterogeneous structure, which was adjusted to a power law distribution. This indicate that most species had a few links, while few species had many more interactions than expected based on random networks with similar properties (Jordano et al., 2003; Gilarranz et al., 2011). This could explain why our networks did not have high nestedness values, compared to other mutualistic networks in disturbed tropical environments (e.g., Bustamante-Castillo et al., 2018; Maruyama et al., 2019), giving it a compartmentalized structure.
In contrast to the quantitative matrices, the network based on presence or absence of coffee plantations followed a nested pattern in which the interaction was organized by a core group of species: S. beryllina, E. poliocerca (two Emerald species notably generalists, Rodríguez-Flores et al., 2019) and C. fragans and M. paradisiaca (specialist and generalist nectar resource, respectively; Bascompte et al., 2003). Once the number of interactions was included, the nested pattern disappeared disclosing the modular organization and denoting the role of these nodes in maintaining the cohesive pattern in this network.
Another explanation for the low nestedness found in land use networks is related to the small size of each network, which has been observed in other studies (i.e., Rivera-Hutinel et al., 2012; Vizentin-Bugoni et al., 2016). Recently, Arizmendi et al. (2021) analyzing the hummingbird-plant interaction network in the same study area, recorded interacting species that were not observed in our study (e.g., Selasphorus rufus, S. platycercus, and S. calliope hummingbird species and Vernonia cordata, Guarea glabra, Ixora coccinea and Dombeya wallichi plant species). They also recorded interactions between network partners (e.g., E. poliocerca and D. wallichi) that we did not observe in our sampling (i.e., missing links; Olesen et al., 2011). Meanwhile, we recorded new interactions in the study area (e.g., between L. brachylophus and C. fragans and between P. mexicanus and Hibiscus uncinellus); only 14 interaction pairs were consistent in both studies. The discrepancies in these interaction records could be attributed to the 10-year time difference between the study periods, plant phenology, and the migratory movements of the species involved. Even when in an interannual analysis, Chávez-González et al. (2020) did not find differences in the network’s connection pattern, they find differences in species composition (i.e., high interaction turnover), suggesting that the presence of hummingbirds and flowers remained highly dynamic over time. In addition, land use changes that occurred between our study and that of Arizmendi et al. (2021) could also determine the interaction dynamics (Dalsgaard, 2020).
Contrary to our prediction, the cattle pasture network was not nested. Similar studies observed higher nestedness in the networks of disturbed areas, compared to primary forests (e.g., Bustamante-Castillo et al., 2018; Maruyama et al., 2019; Díaz-Infante et al., 2020). Generally, the most disturbed sites negatively affect functionally specialized hummingbirds, especially those with long bills, such as hermits and fandangueros (Maglianesi et al., 2015). At such sites, hummingbird species are functionally less diverse, and interaction networks are more nested. Nevertheless, some recent studies have shown that some hummingbird-plant networks are heterogeneous and have low nestedness (Vizentin-Bugoni et al., 2014, 2016; Chávez-González et al., 2020).
In the present study, the modularity values between the types of land use indicated that the late forest and coffee plantation networks were more modular than the cattle pasture network. These results are in line with that obtained by Maruyama et al. (2019) in an urbanization gradient, which is explained by the functional trait diversity present in a biotic community. Studies have shown that, in disturbed environments, the number of hummingbirds with functionally specialized traits decreased (Hardley et al., 2018), resulting in a more generalized interaction networks (Maglianesi et al., 2015; Maruyama et al., 2018). In the present study, in the late forest and shade coffee plantation networks, species interacted more strongly with each other than with other species in some modules. For example, some modules were made up of large, long-billed hummingbirds (i.e., C. hemileucurus, H. constantii, H. longirostris, and P. mexicanus) that frequently visited flowers with long corollas (i.e., C. pictus, H. coronarium, M. paradisiaca, and Heliconia spp.), which were rarely visited by short-billed hummingbird species (i.e., L. brachylophus and T. dupontii). Species that present a morphological mismatch have limitations or prohibited links due to the inability of some hummingbirds to reach the nectar of the flowers (Temeles et al., 2002; Vizentin-Bugoni et al., 2014).
The information on the roles of the species showed that S. beryllina and E. poliocerca were nodes that maintained network cohesion across the different types of land use; as they were highly connected species, they formed generalist nodes. Both species are usually abundant in humid environments throughout their geographic range (Arizmendi and Berlanga, 2014) and tend to present generalist behavior (Rodríguez-Flores and Arizmendi, 2016). In our study, both species were observed actively defending their floral patches or feeding sites and engaging in aggressive behavior, potentially limiting the interaction frequencies of other hummingbird species (e.g., L. brachylophus, T. dupontii) by scaring them away. This behavior pattern has been commonly observed in both hummingbird species, especially in S. beryllina (Rodríguez-Flores and Arizmendi, 2016; López-Segoviano et al., 2018).
Our networks presented intermediate specialization values, a result that contrasts with that obtained by Morrison and Mendenhall (2020), who identified more specialized interaction networks in primary forests than in coffee plantations. However, the plantations they studied seemed to have a significant reduction in tree cover, while the shade coffee plantations in our study maintained an important tree cover, similar to that observed in mature forest (Alvarez-Alvarez et al., 2021). Moreover, the late forests had the highest number of specialist hummingbird species, such as Cynanthus latirostris, P. mexicanus, and L. brachylophus. The latter two species, which are endemic to a small portion of western Mexico (Arizmendi and Berlanga, 2014), played a specialist role in the coffee plantations. This finding highlights the relevant role that these agroforestry systems play in providing resources for the conservation of species with a restricted range and sensitivity to disturbance (Sonne et al., 2016). On the other hand, in the cattle pastures, we mostly observed generalist hummingbirds, such as A. rutila and Archilochus colubris, which are usually tolerant to disturbance (Maruyama et al., 2019). Regarding plants, J. adenothyrsa, C. pictus, and C. fragans were specialists in their relationships with the hummingbirds in the late forests. These plant species were present also in coffee plantations, which had remnant species of the original forest as well as cultivated species (Valencia et al., 2014; Alvarez-Alvarez et al., 2021).
Our networks seem to be fragile in the face of random species loss and the removal of generalist species (Memmott et al., 2004). However, when plants and hummingbirds were eliminated based on threat degree, the networks were more robust. The robustness index measures changes in network structure following a specific extinction scenario but does not consider the rewiring process between nodes; this process improves robustness against node removal (Vizentin-Bugoni et al., 2019). Despite this, our simulations showed that, when the most vulnerable species in the system based on its risk category and endemism level (i.e., L. brachylophus) was eliminated, the network remained robust. This finding could be explained by the low degree of this hummingbird species and their low contribution to network cohesion. However, it does not seem to be consistent with other microendemic hummingbirds, such as Eriocnemis mirabilis, in CFs in Colombia (Ramírez-Burbano et al., 2017), where this species was found to be the most connected in the network.
The cattle pasture network had greater robustness than the late forest and coffee plantation networks because, in cattle pastures, there were more generalist species that are less selective in their ecological links and are more resistant to disturbance (Santos de Araújo, 2018). Thus, our findings suggest that the transformation of primary CFs into intensively managed land uses leads to communities that are functionally less diverse and more generalized interaction networks, which may have contrasting effects on the conservation and robustness of plant-pollinator interactions.
Our results also confirmed the hypothesis that foliage height diversity is the best predictor of the number of hummingbird visits to plants, even greater than the number of flowers, which is consistent with other studies (e.g., Vizentin-Bugoni et al., 2014). In the study area, the late forests and coffee plantations had the most foliage height diversity, hummingbird richness and frequency of tree visits. The coffee plantations had the greatest combination of mature forest trees (i.e., I. vera, S. grandis), shrubs (i.e., Syzigium jambos), and introduced species of commercial interest (C. arabica, M. paradisiaca, and Citrus limonia) that are frequently visited by some hummingbird species (e.g., P. mexicanus, C. hemileucurus). Plant heterogeneity in coffee plantations promotes greater diversity of foliar strata, which not only increases the number of flowers, but also provides perching, shelter, and nesting for different bird species (Chmel et al., 2016), including hummingbirds. Although the role played by foliar strata in plant-pollinator interactions has been insufficiently studied, Klecka et al. (2018), as well, found this variable to be a good indicator of the frequency of visits by different pollinators.
In Amazonian forests, where vertical stratification is remarkable, the hummingbird community is spatially separated; hummingbirds of the Phaethornithinae subfamily are most abundant in the understory, while those in the Trochilinae subfamily dominate canopy interactions (Cotton, 2008). In the present study, there were more flowers in the cattle pastures than in the late forests due to the abundance of pioneer shrubs and herbs with a high density of flowers. However, the number of flowers at these sites did not determine the number of hummingbirds visits to plants, possibly due to the low food rewards offered by these plants. Attributes such as nectar quality may play an important role in hummingbirds’ plant species preferences (Kim et al., 2011).
ConclusionsOur data suggested that, although hummingbird-plant assemblages can persist in managed environments such as shade coffee plantations, the complete conversion of natural habitats to heavily anthropized landscapes can cause drastic changes in biotic interaction patterns. This undoubtedly have implications for the conservation of mutualistic pollination interactions. Shade coffee plantations and late succession forests, however, present hummingbirds with the highest specialization in the use of floral resources, but little tolerance to disturbance. This same specialization results in the networks of both land uses being more modular compared to cattle pastures. This denotes the relevance of certain agroforestry systems such as shade coffee plantations that, by maintaining floristic elements of the original vegetation, promote the permanence of important functional groups in ecosystem dynamics, as was shown with some hummingbirds in this study.
Conflict of interestThe authors declare no conflict of interest
The first author (AIL-F) thanks Conacyt (#930095) for the scholarship granted to carry out her master's studies, as well as the Posgrado en Recursos Naturales y Ecología de la Universidad Autónoma de Guerrero. We thank Daniela Bravo and Diana Poblete for their support in the fieldwork. We also thank the American Bird Conservancy (#1953AM) for financial support to carry out this research. Two anonymous reviewers made substantial contributions to improve an earlier version.