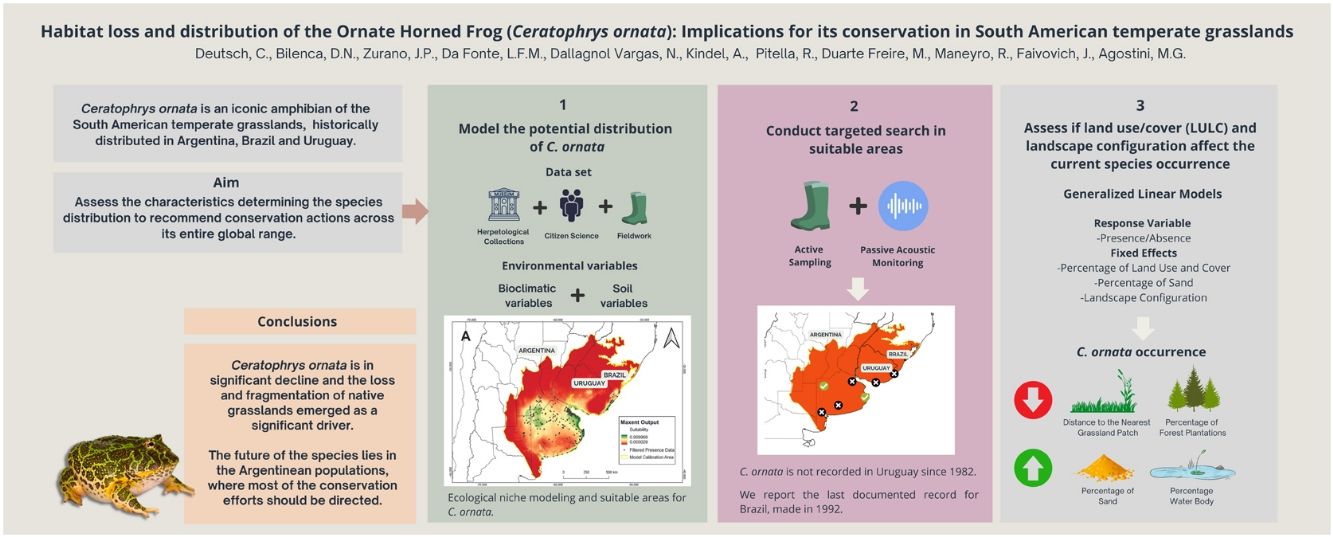
Ceratophrys ornata is an iconic and regionally threatened amphibian of the temperate grasslands from Argentina, Brazil, and Uruguay. Conservation assessments suggested that habitat loss is the main threat to the species, although no studies have yet explored the link between grassland replacement and C. ornata decline. Using a robust database with ∼1000 records from herpetological collections, citizen science, and long-term monitoring, we modeled the species' ecological niche to produce a map of suitable areas, where we searched for C. ornata using active and passive techniques. After exhaustive efforts, we failed to obtain recent records from Brazil and Uruguay. We also conducted Generalized Linear Models to explore the relationship between land use/cover, landscape configuration, and current species occurrence. Overall, results showed that C. ornata occurrence is negatively associated with variables related to native grasslands fragmentation and replacement (i.e., Distance to the Nearest Patch, Forest Plantations), suggesting that habitat loss may have driven local extinctions and population declines of the species. Finally, we outline the regional and national conservation needs of C. ornata and recommend focusing on in-situ conservation strategies for the Argentinean populations to ensure the species' viability.
Rapid rates of biodiversity loss indicate that the Earth is heading toward a sixth major extinction event (Ceballos et al., 2017). Although numerous species from all taxonomic groups are affected, amphibians have experienced worldwide population declines, currently being the most threatened vertebrate group (IUCN, 2023). Since the early 90 s, efforts have been made to document the amphibian crisis, with habitat loss, pollution, and infectious diseases identified as the main causes of decline (Campbell Grant et al., 2020).
Recently, biological surveys to document amphibian biodiversity resulted in rediscovered species, including some that were considered to be locally or globally extinct (e.g., Jaynes et al., 2022; Lopes et al., 2021). Furthermore, wide natural fluctuations in population size over time have been reported for many amphibians (Alford and Richards, 1999; Pechmann and Wilbur, 1994), while community/metapopulation level studies have reported local amphibian extinctions followed by recolonization processes that mitigated or reversed previous decline events (Moss et al., 2021). Therefore, long-term studies focusing on distributional ranges, activity patterns and movement ecology are needed to determine unambiguously whether an amphibian population is suffering an unusual decline (Campbell Grant et al., 2020). On the amphibian decline puzzle, such studies also help to accurately define conservation status and support evidence-based conservation actions (Guisan et al., 2013).
The Neotropical Realm contains nearly half of all amphibian species, and ∼35% of them are threatened or extinct (IUCN, 2023). In line with global trends, habitat loss is suggested to be the main cause of Neotropical amphibian declines (Catenazzi and Von May, 2021). Moreover, the fast and extensive degradation of Neotropical biomes makes the data on species distribution at local level quickly obsolete, highlighting the need to constantly update this information and carefully analyze species absences (De Kort et al., 2020; Wang et al., 2021). Thus, there is critical to investigate distribution patterns to understand the population and species-level associations with habitat loss (Cushman, 2006).
The Ornate Horned Frog (Ceratophrys ornata) is an iconic amphibian inhabiting the South American temperate grasslands (SATG) from Argentina, Brazil, and Uruguay (Carreira and Maneyro, 2019). Over the past three centuries, SATG have been gradually converted into agroecosystems, with different intensities of grassland replacement (Bilenca and Miñarro, 2004). Nonetheless, the introduction in the 90s of GMO glyphosate-resistant crops and the use of no-till technologies have promoted the unprecedented expansion of agricultural frontiers, a process known as agriculturalization (Baeza and Paruelo, 2020). In Uruguay and southern Brazil, SATG were also replaced by significant extensions of exotic forest plantations (Bilenca and Miñarro, 2004). The SATG also host the region's biggest metropolitan areas, concentrated mainly on the coast (Coutinho et al., 2009).
Ceratophrys ornata is listed by the IUCN as Near Threatened with decreasing population trends (Kwet et al., 2004). National assessments considered the species as Vulnerable in Argentina (Vaira et al., 2012) and Uruguay (Carreira and Maneyro, 2019), and as Critically Endangered (Possibly Extinct) in Brazil (MMA, 2022). Although global and national assessments suggest that the major threat affecting C. ornata is habitat loss due to agriculture and urban developments (Carreira and Maneyro, 2019; Kwet et al., 2004; Vaira et al., 2012), there are no studies evaluating the extent of this human-mediated threat on the species. Such studies have been limited by the scarcity of records, likely reflecting a combination of small population sizes with biological characteristics that make C. ornata challenging to detect in the field, and the lack of long-term monitoring at local or regional scales (Deutsch et al., 2017).
In this study, we combined several data sources to obtain occurrence data of C. ornata in Argentina, Brazil, and Uruguay, including ∼1000 records from herpetological collections, citizen science, and long-term fieldwork monitoring. We assessed the environmental characteristics associated with the species potential distribution, evaluated whether habitat loss influenced its current distribution, and recommended actions to conserve C. ornata across its entire global range. We did this by (a) modeling the potential distribution of C. ornata employing ecological niche modeling (ENM), (b) conducting targeted search efforts and performing passive acoustic monitoring and active sampling to obtain novel records in areas with high suitability for the species but without recent records, and (c) assessing if land use/cover (LULC) and landscape configuration after agriculturalization in the SATG affect the current species occurrence.
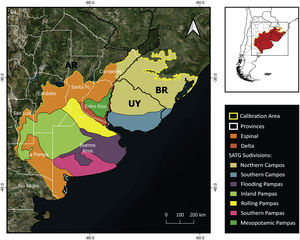
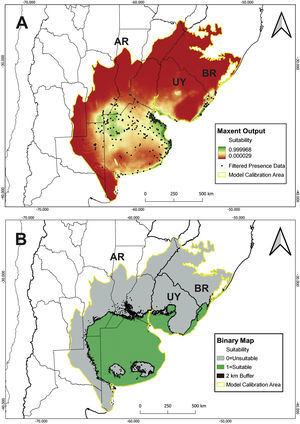
MethodsPotential distribution of C. ornataThe area delineated for the ENM includes the SATG and the Espinal and Delta ecoregions covering the estimated distribution area in Barrio (1980) and IUCN (2008) (Fig. 1) (for more details, see Supplementary material). We used 19 bioclimatic variables from CHELSA V2.1 at the spatial resolution of 30 arc seconds (Karger et al., 2017). Since C. ornata has burrowing habits (Gallardo, 1970), we also used soil layers from SoilGrids (Hengl et al., 2017). LULC layers were not included in the ENM due to the unavailability of layers covering a wide range of record dates. Given the high conversion rate of SATG, modeling the species potential distribution with non-contemporary layers would introduce biases into the model estimation. All variables were clipped to the extent of the selected calibration area (Fig. 1).
We gathered occurrence data from herpetological collections, citizen science, and long-term fieldwork monitoring. To obtain records from herpetological collections, we extensively searched online databases (Supplementary material) and contacted relevant museums, institutes, and universities. We confirmed the record accuracy by visiting the collections or asking curators for photos. Data from citizen science were obtained through a program applied in Argentina, Brazil, and Uruguay (see methodology in Supplementary material). Long-term monitoring in Argentina has been conducted since 2008. Survey techniques included auditory surveys (Heyer et al., 1994) after heavy rainfalls coinciding with high calling activity (Gallardo, 1972). We conducted five auditory surveys (Heyer et al., 1994) per night (1900–0200, 10 min each) over two consecutive nights after heavy rainfall in 240 sites. Some of the records obtained were published in previous studies (Agostini et al., 2012, 2016, 2020, 2021; Deutsch et al., 2017; Perrone et al., 2022).
To model the species potential distribution and suitable areas, we used Maxent 3.4.1 algorithm (Phillips et al., 2017). Niche modeling procedures (i.e., database filtering, correlation analysis, model selection and model conversion to binary) are detailed in the Supplementary material. All statistical analyses were conducted in the R environment (https://www.r-project.org/).
Field surveys in suitable areasIn areas predicted by the model to be suitable for C. ornata but with no recent records (after 1995), we performed targeted field surveys employing active sampling and passive acoustic monitoring (PAM) (Sugai et al., 2019). The site selection and methodological procedure are detailed in Supplementary material. Active sampling, PAM locations and settings of each automatic recorder are detailed in Appendix 1 in Supplementary material. Sites with unsuccessful records were considered as 'absences'.
LULC and landscape configuration associated to C. ornata occurrenceTo evaluate how differently LULC and landscape configuration influence C. ornata occurrence, we employed Generalized Linear Models with binomial family distribution (GLMs) (Crawley, 2007). Due to the lack of high-quality regional LULC layers predating 1985 and considering that our database contains records dating back almost 100 years, we opted for a model incorporating current records and contemporary LULC layers. Hence, C. ornata occurrence was included in a single model as a response variable with two states: presence (records from 2015 onwards) and absence. From each point of presence and absence, we extracted LULC in a buffer of a 1,000 m radius. To define the buffer size, we based on home range studies of C. ornata (Ibáñez, 2023). LULC layers for 2019 were downloaded from Map Biomas Pampa v.1.0. (Baeza et al., 2022). We considered the percentage of six cover categories (Flooded Grassland, Permanent Water Bodies, Agriculture and Livestock, Forest Plantation, Open Forest, and Closed Forest) and of Percentage of Sand in the soil (downloaded from Soilgrids, Hengl et al., 2017), and two landscape configuration variables (Number of Flooded Grassland Patches and Distance to the Nearest Flooded Grassland Patch) as fixed effects. We excluded the Unvegetated Area category from the model since it does not distinguish between urbanized areas, newly planted crop fields and sandy coast. Methodological details of GLM analysis are provided in Supplementary material.
ResultsPotential distribution of C. ornataWe obtained 991 confirmed records (932 for Argentina, 34 for Brazil, and 25 for Uruguay) (Appendix 2 in Supplementary material). To avoid sampling bias and spatial correlation when running the ENM, we used a 3 km buffer, resulting in 317 records.
A total of 9 bioclimatic and soil variables were selected for the model (Table 1). Model building resulted in 48 candidate models with different feature classes–regularization multiplier combinations. Following the sequential criteria (see Supplementary material), the feature class Hinge with a regularization multiplier of 1 was selected as the optimal setting (hereafter H1; MTP OR: 0.00; test AUC: 0.9048; ΔAICc: 89.45). The predicted suitable areas extend to the northwest of Buenos Aires province, east and north of the Atlantic coast (Buenos Aires province), south of Córdoba and Santa Fe provinces, and northeast of La Pampa in Argentina. Suitable areas in Uruguay comprise the southern and eastern coasts and, in Brazil, southern Rio Grande do Sul (Fig. 2A). The binary map predicts that suitable habitat for C. ornata covers about 413,204 km2 (Fig. 2B).
Environmental variables and the values of its percent contribution and permutation importance in the ENM for Ceratophrys ornata.
| Environmental Variable | Description | Percent Contribution | Permutation Importance |
|---|---|---|---|
| Clay | Percentage of clay content | 0.2395 | 0.7741 |
| Sand | Percentage of sand content | 0.2805 | 0.04 |
| Silt | Percentage of silt content | 5.0448 | 2.0295 |
| Mean annual air temperature | Mean annual daily mean air temperatures averaged over one year | 36.0371 | 44.526 |
| Isothermality | Ratio of diurnal variation to annual variation in temperatures | 37.8929 | 22.8015 |
| Mean daily mean air temperatures | The wettest quarter of the year is determined (to the nearest month) | 2.2637 | 0.2421 |
| Annual precipitation amount | Accumulated precipitation amount over one year | 9.8707 | 18.5304 |
| Precipitation seasonality | The Coefficient of Variation is the standard deviation of the monthly precipitation estimates expressed as a percentage of the mean of those estimates (i.e., the annual mean) | 8.1714 | 9.2289 |
| Mean monthly precipitation amount of the warmest quarter | The warmest quarter of the year is determined (to the nearest month) | 0.1996 | 1.8274 |
Most contributing variables are in bold.
Maxent showed that four bioclimatic variables emerged as the most relevant to the model and contributed to 93% of the variation: isothermality, mean annual air temperature, annual precipitation amount, and precipitation seasonality (Table 1).
Field surveys in suitable areasWe obtained 120 absences in areas predicted to be suitable but with no recent records (Appendix 2 in Supplementary material). Active surveys covered ∼368 days/307 h, and ∼808 h of recordings were obtained from passive acoustic monitoring.
LULC and landscape configuration associated to C. ornata occurrenceGLM results indicated that the current occurrence of C. ornata is associated with four variables (R2 Nagelkerke = 0.455): Distance to the Nearest Patch, Forest Plantations, Permanent Water Bodies, and Percentage of Sand. Percentage of Sand (Z = 5.850; p < .05) and Percentage of Permanent Water Bodies (Z = 2.121; p < .05) significantly and positively influenced the species probability of occurrence. Conversely, the percentage of Forest Plantations (Z = −2.216; p < .05) and Distance to the Nearest Patch (Z = −2.297; p < .05) negatively influenced the presence of C. ornata (Table 2).
Summary of the generalized linear model showing the influence of different types of LULC and landscape configuration on C. ornata occurrence.
| Explanatory variable | Estimate | Std. Error | Z value | p |
|---|---|---|---|---|
| Flooded Grasslands | −0.289 | 0.178 | −1.622 | 0.102 |
| Number of Patches | 0.178 | 0.135 | 1.316 | 0.186 |
| Distance to the Nearest Patch | −0.463 | 0.201 | −2.297 | 0.020 |
| Open Forest | 0.135 | 0.129 | 1.044 | 0.306 |
| Closed Forest | −0.272 | 0.146 | −1.856 | 0.064 |
| Permanent Water Bodies | 0.213 | 0.100 | 2.121 | 0.035 |
| Agricultural and Livestock | −0.002 | 0.157 | −0.015 | 0.985 |
| Forestry Plantation | −0.449 | 0.202 | −2.216 | 0.027 |
| Percentage of Sand | 0.93 | 0.160 | 5.850 | <0.001 |
Significant explanatory variables are in bold.
In this study, we compiled information from more than 15 years of field surveys combined with different data sources and sampling techniques to assess the current distribution of C. ornata and the attributes associated with its occurrence. Outstanding results emerged from the citizen science program, revealing new documented records for 1991 and 1992 in Brazil and confirming the occurrence of C. ornata in several historical Argentinean localities. Novel alternative monitoring techniques (e.g., citizen science, eDNA, trained scent dogs) have proven to be successful for the study/rediscovery of declining, elusive and/or rare species such as C. ornata (Bennett et al., 2020; Deutsch et al., 2017; Lopes et al., 2021). In agreement with these studies, we recommend diversifying sampling techniques to complement museum collection data, and overcome the limitations of direct-observational survey techniques which are inherently biased in many aspects and hence can lead to inaccurate predictions.
Our study demonstrated that the distribution of C. ornata is restricted to SATG, with two confirmed records in ecotonal areas of the Argentinean Espinal Region. Although covering a large extension, the species occurrence is not homogeneous, and suitable areas comprise nearly 50% of the SATG. Moreover, the lack of recent records in Brazil and Uruguay could indicate a significant distributional retraction.
GLM results showed that the current occurrence of C. ornata is positively associated with Permanent Water Bodies. After heavy rainfalls, the surrounding areas of permanent water bodies turn into flooded shallow wetlands, providing the breeding habitats preferred for C. ornata (Gallardo, 1970). The Percentage of Sand also emerged as a significant variable positively associated with the current species occurrence. Since C. ornata is a fossorial frog (Gallardo, 1970), we can expect that loose sandy soils could provide favorable burying sites. Nevertheless, the Percentage of Sand was not influential in the obtained potential distribution modeled in Maxent (involving historical and current records) (Table 1). Therefore, we argue that the current occurrence of C. ornata in areas with sandy soils may be related to land uses rather than natural history traits or habitat preference. Considering that sandy soils do not provide proper conditions for profitable agricultural activities (cropping and cattle-raising), these are less impacted than areas with other types of soils (León et al., 1984). This could determine the current occurrence of C. ornata remnant populations in marginal areas of the Flooding Pampas and Inland Pampas. Nevertheless, we should consider that the observed differences may also be attributed to the use of different algorithms (i.e., Maxent and GLM).
Our results also revealed a negative association between C. ornata occurrence and variables expressing the replacement and fragmentation of grasslands (Distance to the Nearest Patch and Forest Plantations). The presence of the species near grassland patches indicates an association with moderately conserved grasslands (see discussion above). Forest plantations also emerged as good predictors of the species absence. Over the past decades, native grassland areas have been replaced by introduced exotic forest plantations in Uruguay (Baeza et al., 2022) and southern Brazil (Lima-Toivanen, 2012), modifying key areas of the species' historical distribution. The decline and local extinction of C. ornata is likely multifactorial and cannot be fully determined in this study. Nevertheless, according to our results (Table 2), the afforestation could have contributed to the decline process, particularly in Brazil and Uruguay. SATG have lost at least 40% of its original cover due to land use change (Baeza et al., 2022; Paruelo et al., 2022), and this study adds evidence to previous ones, linking habitat loss with detrimental effects on grassland specialist species (Claramunt et al., 2022; Codesido et al., 2013).
Contrary to our expectations, we found no significant effects of the variable "Agricultural and Livestock Area" on the species occurrence. However, this variable does not distinguish between crops (e.g., soy, maize, wheat, rice) and pastures, representing an important limitation of the layers used in our study. This could be an essential issue to assess in future studies since it is known that amphibians respond differently to the effects of land use and several of these responses are species-specific (Cushman, 2006).
Finally, it is important to note that national assessments have suggested urbanization and rice crops, particularly in Brazil, as responsible for the C. ornata decline (Carreira and Maneyro, 2019; ICMBio, 2023). The limitations of our study, related to available layers, do not allow us to be conclusive about the effects of these land uses/covers on the C. ornata occurrence.
Conservation implicationsThe impact of habitat loss has been widely identified as the most direct cause of global biodiversity annihilation (Ceballos et al., 2017). However, it has been challenging to determine the association between this threat and decline events, particularly for species with wide distribution ranges (Staude et al., 2020). Furthermore, several studies on amphibian populations in SATG revealed that various factors proposed to explain population declines exhibit a high degree of temporal and spatial variation (Agostini et al., 2020, 2021; Perrone et al., 2022), and this may also be the case for C. ornata. Therefore, given the different scenarios, distinct conservation actions are needed in each country of the species distributional range.
In Uruguay, the historical localities of C. ornata have been extensively transformed, mainly by forest plantations and urbanization (Baeza et al., 2022). Despite our intensive search efforts conducted in key areas, added to previous studies (Gambarotta et al., 1999; Bardier and Maneyro, 2015), the species has not been found since 1982. We recommend intensifying search efforts in coastal wetlands on the west coast of Uruguay, which has not yet been properly explored (Langone pers. comm.). We suggest updating the national conservation status and up-listing the category from Vulnerable to Critically Endangered (Possibly Extinct).
In Brazil, the citizen science program obtained more recent documented records than the last scientifically reported for this country by Braun and Braun (1980) and Gayer et al. (1988). In addition, in the southern State of Rio Grande do Sul, there are extensive suitable areas for C. ornata with some degree of protection (e.g., Taim Ecological Station) that still could harbor remnant populations. We recommend intensifying search efforts in strategic remote areas using passive acoustic monitoring.
To discuss the status of C. ornata in Argentina, it is necessary to perform a regional analysis. The northeastern Buenos Aires Province (also known as the Rolling Pampas subregion, see Fig. 1) emerged as a suitable area. Moreover, museum collections contain many specimens collected in this subregion before 1975, indicating the species was relatively abundant in the past. Nonetheless, the absence of records during field monitoring and the scarcity of recent citizen science records strongly suggest a local population decline in the Rolling Pampas. It is worth mentioning that the Rolling Pampas is one of the most modified areas of Argentina and one of the world's most impacted, where GMO glyphosate-resistant soy crops have replaced more than 75% of the native vegetation (Baeza and Paruelo, 2020). Under this scenario, habitat loss could have affected C. ornata populations and likely explain the remarkable disparity between historical and current occurrences (see Appendix 2 in Supplementary material).
In agreement with previous studies (Deutsch et al., 2017), we identified two areas in the Argentinean territory harboring the best-preserved C. ornata populations and emerging as key areas for ensuring the viability of the species. The two priority conservation areas belong to the Inland Pampas and the Flooding Pampas, respectively (see Fig. 1). The fact that these areas have received moderate modification pressure, conserving the last and largest remnants of native grassland (Baeza and Paruelo, 2020) could explain the high number of current records. Nonetheless, different human activities may compromise the future of the grassland remnants and their biodiversity. In the Inland Pampas, the traditional land use consisting of extensive cattle ranching has been rapidly converted to crops in recent years, reducing the number and extension of natural grassland patches (Baeza et al., 2022). In the eastern part of the Flooding Pampas, urbanization and intensive cattle-raising have recently expanded over the grassland remnants (Athor and Celsi, 2016). Urbanization and tourism development promote the existence of forestations of invasive exotic species, road and trail construction, and wetland drainage, which are carried out without ecosystem planning (Athor and Celsi, 2016). We argue that applying in-situ conservation actions, combining research, mitigation activities and educational and awareness-raising programs could present a viable and practical approach to conserving C. ornata in Argentina. Remnants of natural grasslands and associated wetlands must receive conservation priority and increasing protected areas coverage (private and public) could offer an opportunity to conserve grassland patches. This is particularly suitable for the Flooding Pampas, where other conservation strategies that recognize and encourage alternative economic uses like sustainable cattle-raising (e.g., Alianza de Pastizal; Altmann and Berger Filho, 2020; Vaccaro et al., 2020) have been extensively working in the same direction. Furthermore, we recommend moving forward on population genetics studies that will allow us to evaluate future in-situ and ex-situ population management. Finally, productive activities and urbanization will continue to be in constant conflict with the conservation needs of temperate grassland relicts. In agreement with the conservationist and academic community, we highlight the need for better policy legislation to control human activities advancing on the remnants of native grassland and associated wetlands. These actions will undoubtedly benefit not only C. ornata populations but also the threatened biodiversity of the South American temperate grasslands.
ConclusionThis study reveals important aspects of C. ornata distribution to guide conservation actions in the temperate grasslands of South America. Ceratophrys ornata is in significant decline and the loss and fragmentation of native grasslands emerged as major drivers. Given the current knowledge synthesized in this study, the future of the species lies in the Argentinean populations, where most of the conservation efforts should be directed.
Conflict of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Funding: National Geographic Society (NGS-56065C-19), the Rufford Foundation, Amphibian Survival Alliance (5362-ASA) and Neotropical Grassland Conservancy. GA and DB were funded by ANPCyT (PICT 2018-00839) and UBACyT (20020190100244BA). JF was funded by ANPCyT (PICT 2019-346) and CONICET (PIP 2021-2023 11220200102800CO). RM was partially funded by ANII and PEDECIBA (Uruguay). RAN-ICMBio partially funded the field efforts in Brazil. We thank Sofia Perrone, Isis Ibáñez, Alexis Navarro, Mauricio Saavedra, Lorena Barranco, Santiago Nenda, Matías Olmos, Jamil Corrêa Pereira, Jaqueline Goldani Becker, Melina Peluffo, Paula Laporta, Pablo Otero, José A. Langone, Dante Roibal and ONG Aguará Popé. We also thank all the curators and technicians of the herpetological collections listed in Appendix 2 in the Supplementary material for providing specimen data information. Finally, we would like to extend special thanks to the more than 1000 active participants of the citizen science program 'Giant of the Pampas' who generously provided species occurrence data used in this study.