Disturbances are key events in ecological systems, strongly impacting biological communities. This study disentangles the effects of a chronic (flood) and acute (fire) disturbance to determine their interactive effects on ant species richness and composition at different temporal scales. For this, we sampled ground-dwelling ants with pitfall traps on native grasslands in the Pantanal wetland, one of the world's largest floodplains. We sampled ants ten days (short-term), one year (medium-term), and four years (long-term) after fire and at varying elevations (a surrogate for the time that these areas remain flooded). We found that fire was the dominant factor in determining ant community patterns in flooded areas. In the short-term, fire substantially changed species composition and increased species richness. However, these effects decreased at one year and disappeared after four years, demonstrating the ant community’s resilience. Elevation and, consequently, flooding, did not influence any measured parameter, indicating that the ant species are adapted to colonize other areas rapidly or survive in such flooded habitats. Our results suggest that Pantanal ant communities can recover from acute fire disturbances after some years. However, increasing fire frequency caused by global climatic changes and recent Brazilian environmental policy misconducts would probably compromise the observed resilience.
Natural or anthropogenic environmental disturbances are key events in terrestrial and aquatic systems that may influence the structuring of populations, communities, and ecosystems (Mouillot et al., 2013). Their effects on communities can be seen across different temporal and spatial scales (Turner, 2010). The type, frequency, and intensity of disturbances determine the extent to which communities are affected and their ecological resilience following each disturbance (Tanentzap et al., 2014). Disturbance events can act as extinction filters (Drapeau et al., 2016), and the type and extent of the disturbance can be of great importance for the strength of these filters (Betts et al., 2019). The resistance and resilience of communities to disturbances can vary among taxonomic groups and the local environmental conditions, reinforcing the context-dependency of biological responses to disturbances (Garnier et al., 2017). Despite the importance of disturbances for community organization and the variety of biological responses, most studies are limited to immediate temporal effects and focus on determining the effects of disturbances in isolation, rather than considering the interactive effects of multiple disturbance types (Kéfi et al., 2019).
The effects of different disturbances on biological communities can be classified as dominant, multiplicative, or additive when evaluated independently and as synergistic or antagonistic when considered interactively (Côté et al., 2016). If more than one disturbance impacts the community and the total result of these disturbances is the same as the sum when individually measured, the effect of the disturbances is classified as ‘additive’ (Côté et al., 2016). In contrast, synergistic disturbances have a greater combined effect, and antagonistic disturbances produce a weaker combined effect than if those disturbances occur as isolated incidents (Folt et al., 1999).
Most disturbances with the potential to change biological patterns do not occur in isolation (Graham et al., 2011). Despite this, some disturbances are more often studied as isolated events, such as floods (Oliveira et al., 2014) and fires (Karavani et al., 2018). These disturbances are often associated with the expansion of anthropogenic activities, such as increases in human induced fires, and climate change. Thus, studies evaluating the response of biological communities to the combination of these disturbances are necessary to understand the full effects of anthropogenic change on our ecosystems (Oliveira et al., 2014; Paolucci et al., 2016).
Floodplains are riverbank ecosystems subject to periodic flooding during the rainy season (Tomas et al., 2019). They have high productivity and rich biodiversity that support vital ecosystem services locally, regionally, and globally, such as ecotourism, river flooding mitigation, and habitat for rare and endangered species (Mitsch et al., 2015). Previous studies have shown that the period for which these areas are flooded, a possible indicator of the disturbance intensity, directly impacts terrestrial communities (Poff et al., 2018; Tanentzap et al., 2014). Rainfall has an important effect on floodplain communities, yet there is growing evidence in the literature that other disturbances, such as fire, also have significant effects on these ecosystems (Kleindl et al., 2015). Fires are becoming more frequent over time, especially those of anthropic origin (Thielen et al., 2020).
In the Pantanal biome, one of the largest freshwater floodplains in the world (Harris et al., 2005), floods are constant disturbances, shaping the vegetation structure and their organisms for over 2.5 Ma (Assine and Soares, 2004). The Pantanal is highly seasonal, and during the wet season, a vast amount of its plains are covered with water, while most of these plains dry completely during the dry season, when most fire events occur (Nunes da Cunha et al., 2006). While natural fires occur in the Pantanal before human occupation (Power et al., 2016), cattle ranchers have historically used them for pasture management at small spatial scales (Junk et al., 2006). Recently, however, the number and extent of anthropogenic fires have dramatically increased, breaking record after record (WWF, 2020), probably caused by recent changes in Brazilian environmental policy. Indeed, the fire events in the Pantanal changed from natural or low human manipulation disturbances to less predictable and intense disturbances (Pivello et al., 2021). Therefore, in such extreme scenarios, the Pantanal fire events can be considered a more acute and unpredictable disturbance than the seasonal floods (chronic disturbances).
Ants are widely used to evaluate the effect of environmental disturbances, and their responses to different disturbance types can vary at different spatiotemporal scales (Andersen, 1999). For example, some previous studies have shown that fire changes the structure of ant communities even after a few years (Maravalhas and Vasconcelos, 2014; Parr et al., 2004). However, rapid community recovery (less than a year) has been observed in other studies in savannas and semiarid regions (Andersen et al., 2014; Parr and Andersen, 2006). In floodplains such as the Brazilian Pantanal, ants of flood zones are constantly subjected to flood disturbances (Dambros et al., 2018). Depending on the elevation of a given location, the flood period may vary from days to months but are generally predictable, occurring seasonally (Junk et al., 2006). While the Pantanal ant communities face two kinds of disturbance, with different impacts and predictability, their response to these joint disturbances is still poorly known.
Although ants may respond differently to fire and flooding in the Pantanal, these responses can be related to time since these disturbances. Most ground-dwelling ant species forage on the trees and surrounding vegetation or change their foraging territories to upper elevations during a flood (Adis, 1997). However, after the plains dry out, these ants return to their usual foraging sites in the soil/ litter strata, now with a simplified structure, which may force them to increase their foraging rates. Indeed, a similar situation happens soon after a fire, and ground-dwelling ant colonies often increase their activity rates (Parr et al., 2007) also due to a simplified habitat and a decrease in the abundance of litter-dwelling arthropod prey (McGlynn et al., 2003). These two factors combined can force the ant colonies to allocate more foragers and forage longer distances (Sanders and Gordon, 2002), and this should be even more intense in the already simplified dry floodplains.
After a longer period, the main effects of the disturbances are not on ant activity but rather on their diversity patterns and community structure (Andersen, 2018). Although the Pantanal ant communities are presumably adapted to seasonal floods and occasional low-intensity fires, the same may not be true to high-intensity and unpredictable human-induced fires. Since chronic disturbances often act as an environmental filter to ant communities, we may expect their effect to be perennial, leading to uniquely adapted ant communities. However, we should expect the ant community to have a more intense response to acute disturbances, at least temporally, such as fires, which can extirpate less adapted species and change overall species coexistence patterns (Rosa et al., 2021). Therefore, if there is a synergistic effect of fire and flood on ant communities, it should be lowered with time, as the community recovers from the acute disturbance.
Here, we assessed the effects of acute and chronic disturbances in Pantanal ant communities, asking whether these disturbances synergistically affect these communities. We expected the fire effect (acute disturbance) to be punctual and to disappear over time, while the flood effect (chronic disturbance) would remain constant, thus: (1) the effects of both (flood and fire) will act synergistically in the short-term post-fire, positively affecting the number of local species and changing ant community composition; (2) in the medium-term post-fire, we still expected a synergistic effect, but with a decreased effect of fire on community richness and composition; (3) in the long-term, the post-fire community would stabilize, and only the effect of flood would be observed on ant community richness and composition.
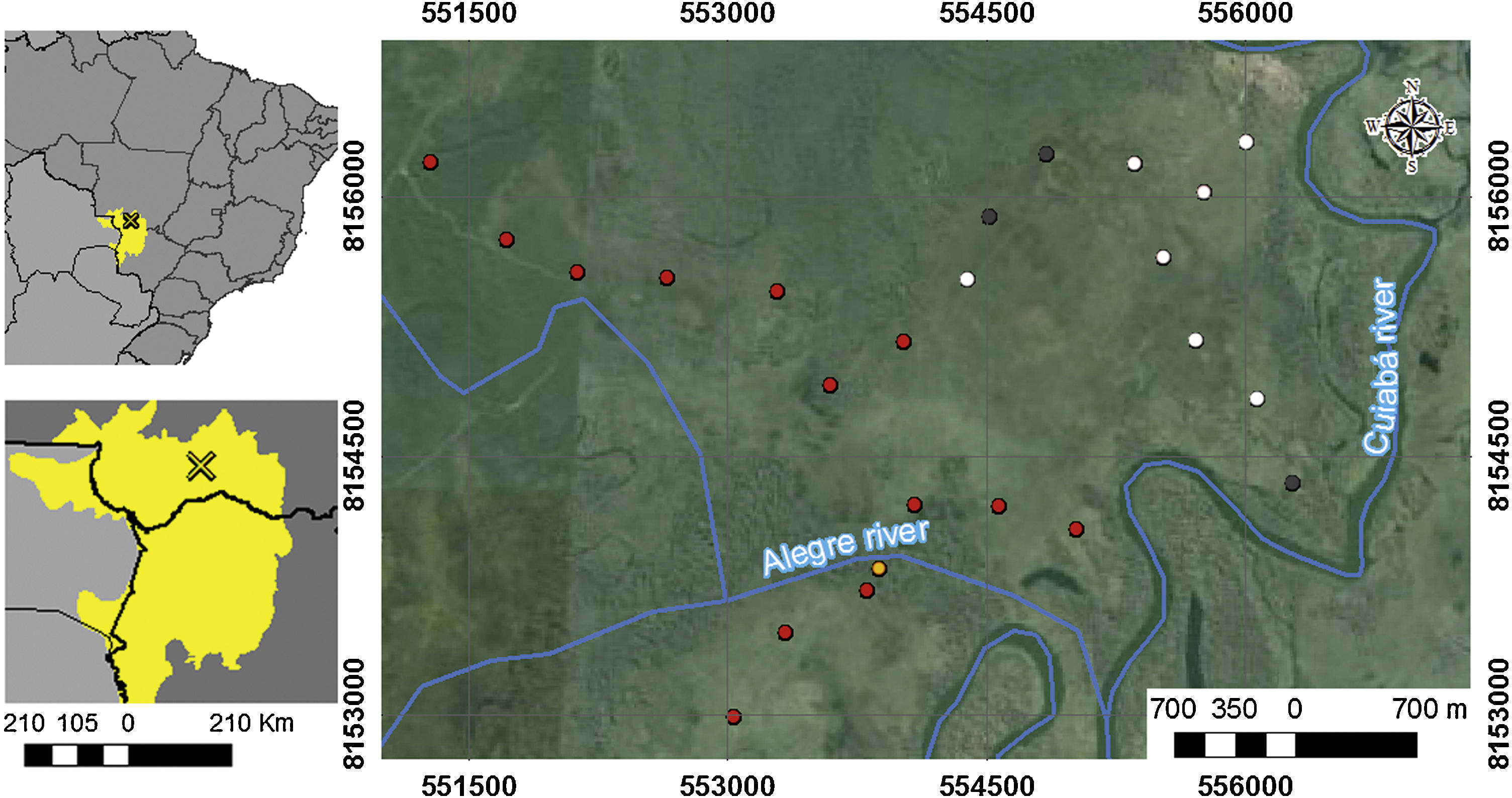
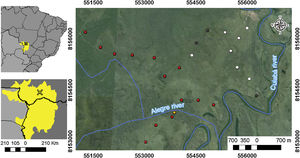
Material and methodsStudy areaSampling was conducted at São Sebastião do Borba farm (16°40ʹ15.92ʺS, 56°28ʹ24.44ʺW), located in the municipality of Poconé, State of Mato Grosso, Brazil. The farm is located in the floodplain of the Cuiabá River, in the Pantanal area of Poconé (Fig. 1). The climate is AW, hot and humid under Köppen’s classification, with a mean annual temperature of 25 °C, and a mean annual rainfall of 1250 mm (Signor et al., 2010). The seasonality is typical of the Pantanal, with a dry season ranging from May to September and the wet season from September to April (Junk et al., 2006). The highest flooding level occurs between January and February, reaching a water depth of approximately 90 cm at the peak of the flooding season (Nunes da Cunha and Junk, 2001). The difference between areas that remain flooded for a few days or months is determined by only a few centimeters in the relief (Junk et al., 2006).
Study area location: (A) Distribution of the Pantanal biome (in yellow) in the countries of Brazil, Paraguay and Bolivia; (B) location of the Pantanal of Poconé, in State of Mato Grosso, Brazil; and (C) location of the 24 plots (points) used for sampling epigeic ants in relation to the rivers that cross the area. White points represent areas that were not burned, gray points were burned only in 2011, orange points areas burned only in 2015, and red points represent areas burned in 2011 and 2015.
The vegetation of the Pantanal consists of several mixed forest habitats and a seasonally flooded mosaic. We sampled ants on native grasslands, consisting of different shrubs and herbs and young individuals of pioneer tree species (Nunes da Cunha et al., 2006). These grasslands are located in low terrains with little altitudinal variation (da Silva et al., 2020). Native grasslands are dominated by plant species of the genus Combretum (Nunes da Cunha and Junk, 2001), which impose difficulties for cattle ranching, and fire is used to manage these shrubs and pasture formations (Libonati et al., 2020). These illegal fires often spread beyond the intended area, reaching other adjacent areas (Layme et al., 2012). The fire history in the study area is well known, and during the dry seasons of 2010, 2011, and 2015, illegal fires burned specific portions of the farm that were later used for data collection in this study.
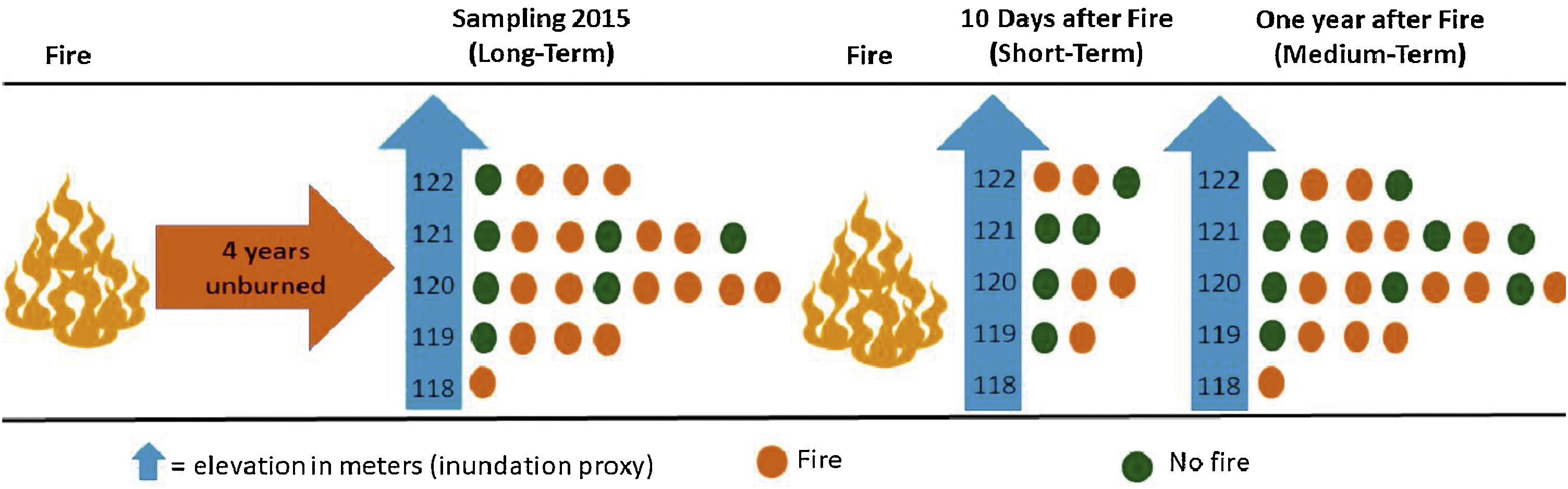
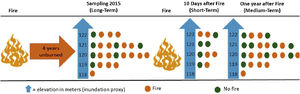
Experimental designWe evaluated the effects of flood and fire in areas with different fire histories (short, medium, and long-term), and elevation was used as a proxy for inundation (see details below). To evaluate the effect of fire, we collected data to observe the short, medium, and long-term effects of fire. We sampled ant communities three times, twice in October 2015, first before a fire occurred in the region (2015 pre-fire) (n = 24 sampling points) and again 10 days after the fire (2015 post-fire) (n = 10). The decrease in the number of samples after the fire was due to logistical constraints since the fire was an unexpected event. Samples were collected a third time in October 2016 (2016 post-fire) (n = 24), a year after the 2015 fire. These points are distributed within approximately 1500 ha, with an average distance of 450 m between adjacent points (Max: 625 m; Min: 130 m). We tested for spatial autocorrelation using the Moran index using the ‘correlog’ function from the ‘pgirmess’ package (Giraudoux, 2021). At each sampling point, we installed 10 pitfall traps at least 20 m apart within a 60 × 80 m plot. These pitfall traps were plastic 500 ml containers (15 cm in diameter) filled with about 50 ml of water and mild detergent. The traps remained active in the field for 48 hours. We measured the terrain altitude above sea level for each sampling point using the Garmin GPS model GPSMAP 64. The measurements were posteriorly double-checked using satellite images (Landsat 8) analyzed with the QGIS (2019). The direct assessment of flood period by satellite images overlay at a fine-scale is infeasible due to the large frequency of clouds during the rainy season. The sampling points had altitudes ranging from 118 to 122 m, representing considerable variation within the range predicted for local studies in the Pantanal, where 1 meter of altitude represents a great difference in the time of flooding (Damasceno-Junior et al., 2005; de Almeida Souza et al., 2019). The elevation of sampling points was used as a proxy to infer the flood period, based on the assumption that higher-elevation areas are subject to shorter periods of flooding. In 2015, the highest sampling points remained flooded for about 60 days, while the lowest ones remained flooded from early December 2015 to June 2016 (∼6 months). As the farm owner has had to move the cattle among areas because of the flood, the information regarding flood duration in the study areas is quite precise. Additionally, it is important to highlight that all sampled areas are flooded every or nearly every year (Pereira et al., 2021). All ants collected were identified at the genus level using the key of Baccaro et al. (2015), then sorted into species/ morphospecies. The specimens were deposited in the Zoological Collection of the Universidade Federal de Mato Grosso, Cuiabá, Brazil.
Short-term effectThe samples collected in 2015 post-fire were used to test the effect of the short-term fire. After 10 days of the fire event, we selected 10 locations, being five points in burned areas and five in unburned areas. The locations in either group did not differ in their history of fire incidents since 2010; none of the locations had experienced more than one instance of fire in 2010 and 2011, and none had burned since 2012 (Fig. 2).
Flowchart representing the sampling methodology divided into three steps (short-term, medium-term and long-term). Short-term: 10 points located in areas burned 10 days before (n = 5) and in unburned areas (n = 5); medium-term: 24 points located in areas burned one year before (n = 14) and unburned points (n = 10); long-term: 24 points located in areas burned four years before (n = 17) and unburned points (n = 7). The points were distributed at five different elevations, which were used as a proxy for flood duration.
We used the 2016 post-fire data, a year after the last fire in the area. For this, we compared the community patterns among the points burned a year before (n = 14) and unburned points (n = 10) (Fig. 2).
Long-term effectTo evaluate the long-term effect, we used the data obtained in 2015 before the fire (2015 pre-fire) and compared the ant community patterns among points located in burned (n = 17) and unburned areas (n = 7) by the fires of 2010/2011 (Fig. 2).
Data analysisWe performed three independent Generalized Linear Models (GLMs) for each temporal scale using the terrain elevation as factor. Thus, we assessed the variation in the number of species in relation to the terrain elevation, the fire occurrence (categorical variable), and frequency of occurrence of ants. To control data overdispersion, we used a negative binomial distribution with the glm.nb function from the MASS package (Venables and Ripley, 2002). Since we have ten pitfall traps (subsamples) in each sample, the frequency of occurrence of each species in subsamples varies from 0 to 10.
We used a Permutational Multivariate Analysis of Variance (PERMANOVA) with 999 permutations to test whether there is an effect of fire (with and without fires as levels) and flood (elevations of points as levels) on the species composition for each of the temporal scale individually, with the terrain elevation and the fire occurrence as factors, using the “adonis” function from the vegan package (Oksanen et al., 2019). The PERMANOVA was performed using dissimilarity matrices (Bray Curtis coefficient) of the composition and frequency of species obtained from the abundance matrix after Hellinger transformation as response variables. To visualize the results, we use the ordination biplot produced by the Non-Metric Multidimensional Scaling (NMDS). All analyses were performed in the R software (R Core Team, 2016).
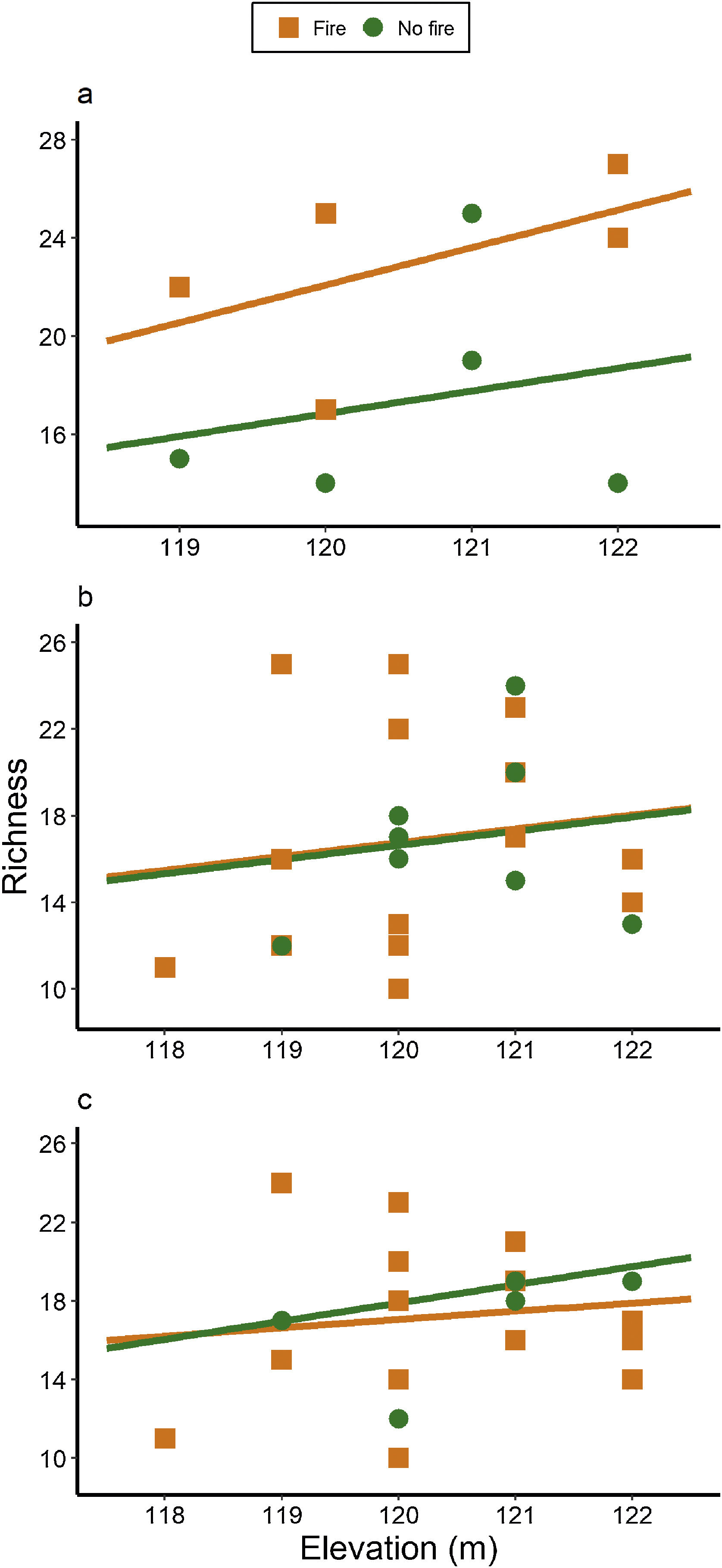
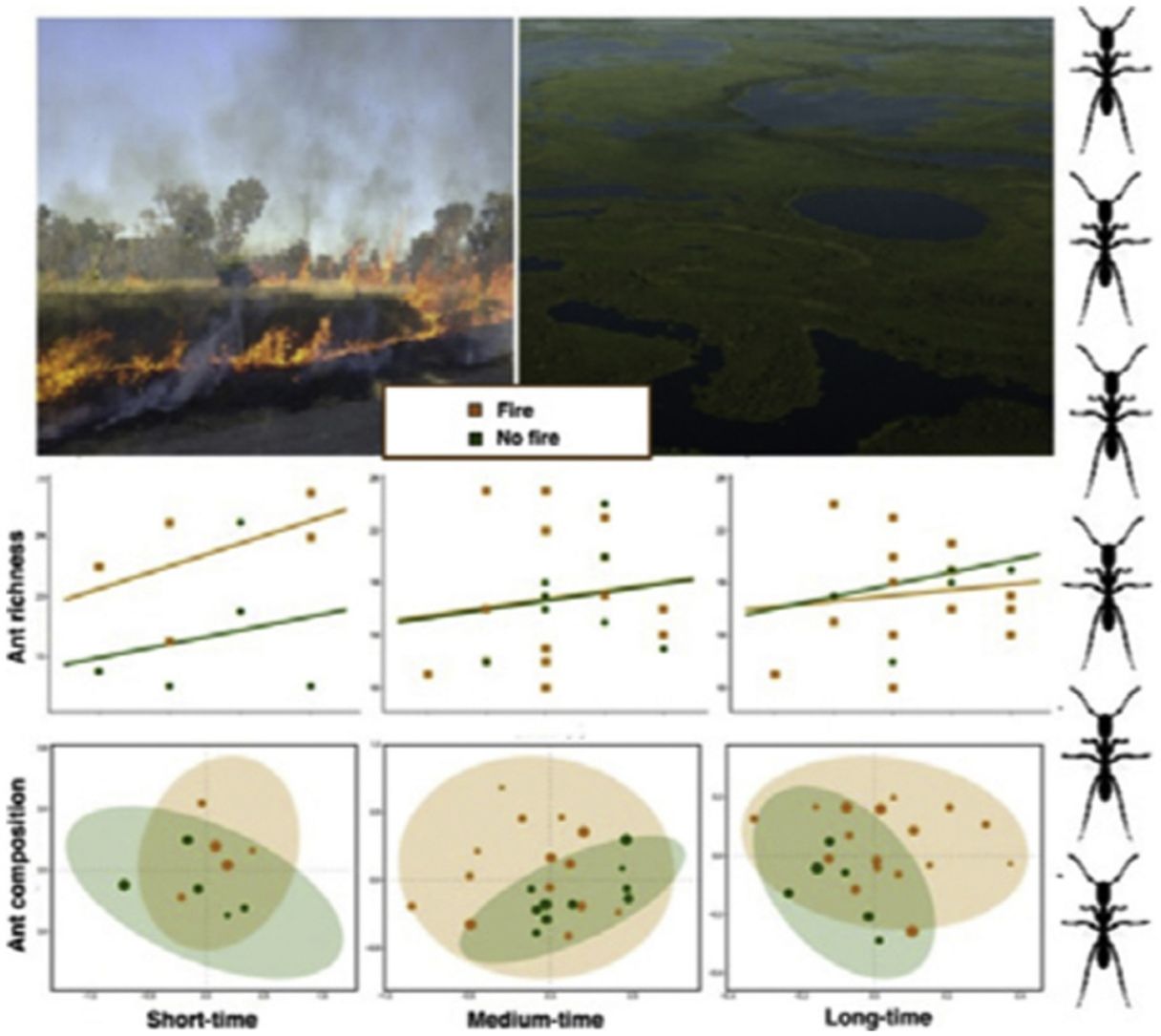
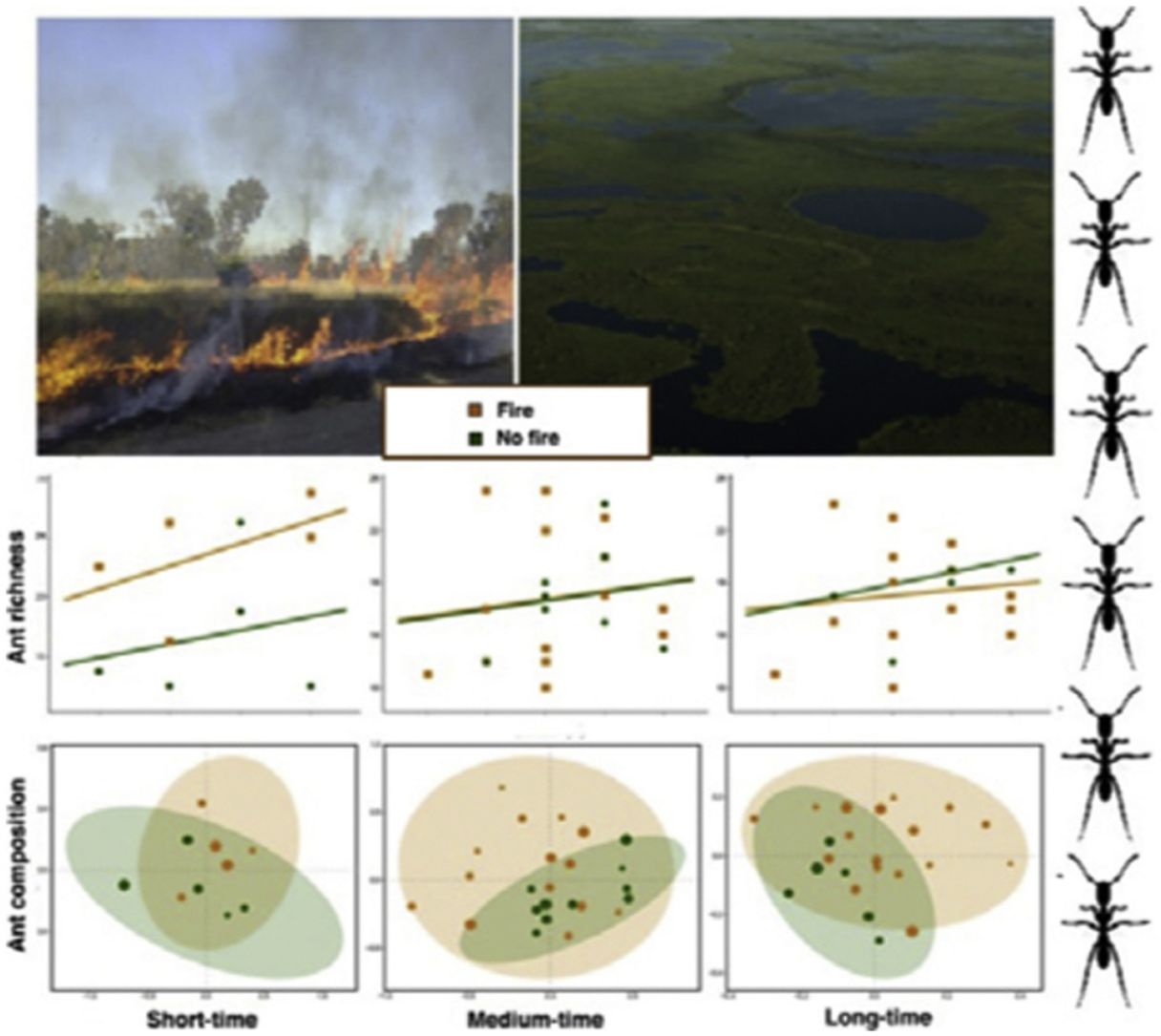
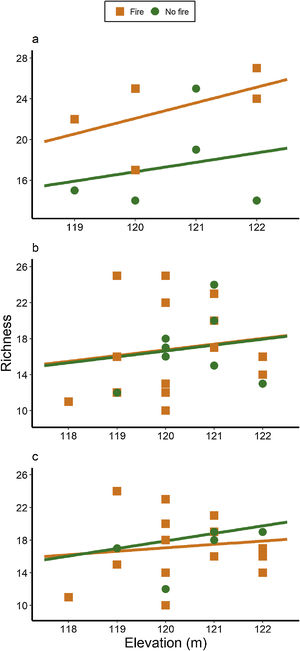
ResultsAnt species richnessWe found a total of 64 ant species in 26 genera and seven subfamilies (Table S1). The most common ant species were Solenopsis sp. 2, Pheidole radoskowskii, and Nylanderia fulva. There was no interaction between fire and flood in relation to species richness in any of the three temporal scales (short-, medium- and long-term) (F1,4 = 1.413, p = 0, 646; F1,18 = 5.529, p = 0.876; F1,18 = 3.110, p = 0.873, respectively). In the short-term, species richness was influenced by fire (F1,4 = 7.472, p = 0.048) and by the local frequency of species occurrence (F1,4= 1.623, p = 0.0272), but not by elevation (F1,4 = 6.496, p = 0.323) (Fig. 3A). Species richness was greater in points subjected to fire and with a higher frequency of species occurrence than in unburned points with a lower frequency of occurrence, regardless of elevations. In the medium and long-term, only the frequency of occurrence influences species richness (F1,18 = 5.554, p < 0.01; F1,18 = 3.136, p < 0.001, respectively), without effect of fire (F1,18 = 27.736, p < 0.886; F1,18 = 18.680, = 0.507, respectively) or flood (F1,18 = 27.106, p = 0.427; F1,18 = 18.243, p = 0.508, respectively) (Fig. 3B,C).
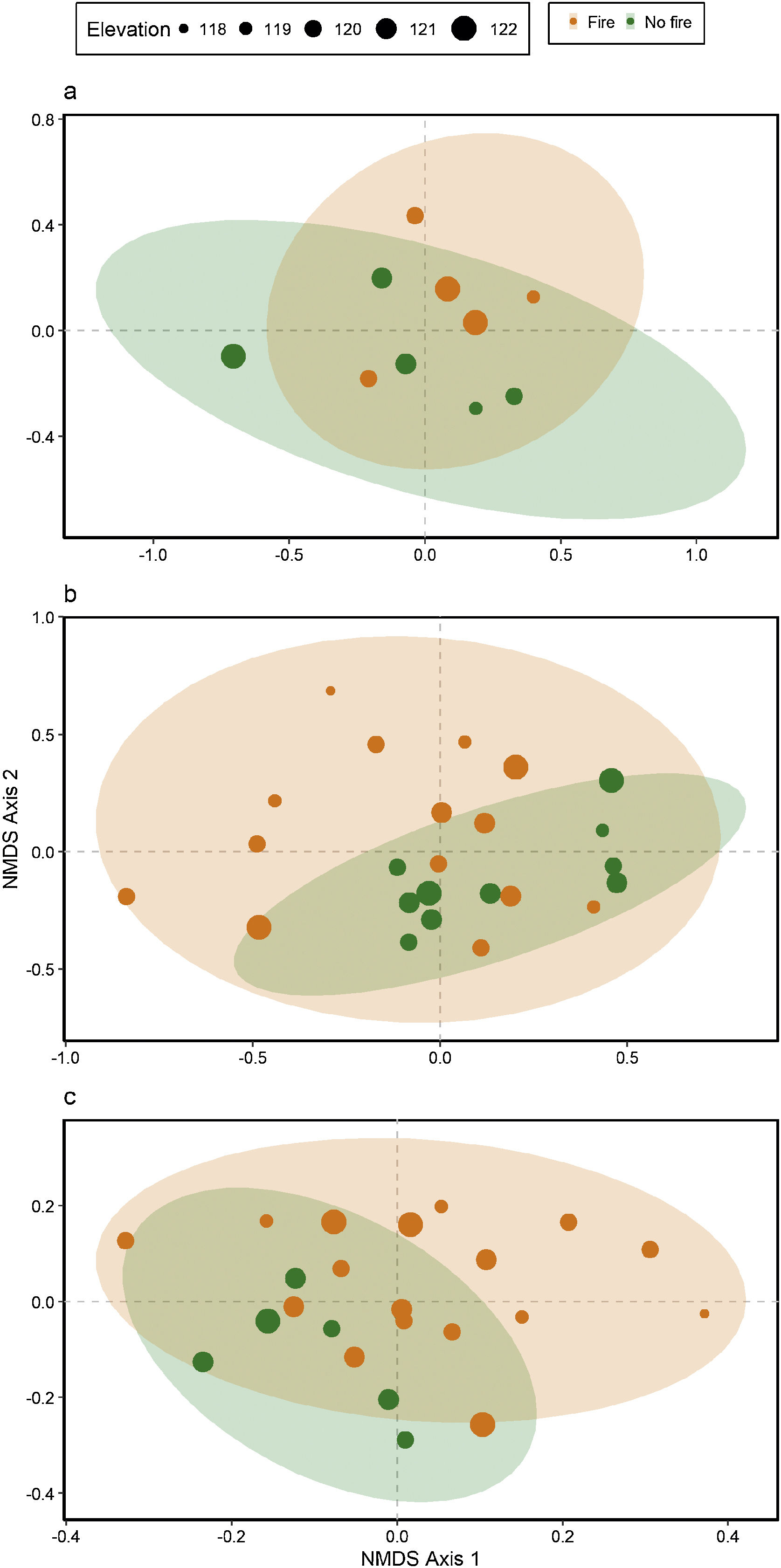
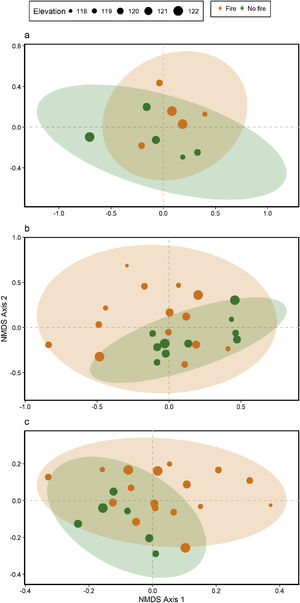
Ant species compositionNo interaction was detected between fire and flooding regarding species composition in any of the three temporal scales (short, medium and long-term) (R² = 0.128, p = 0.204; R² = 0.04489, p = 0.333; R² = 0.132, p = 0.464, respectively) (Fig. 4, Table S2). However, the species composition was influenced by fire in the short and medium-term (R² = 0.181, p = 0.019; R² = 0.09, p = 0.007, respectively), but not in the long-term (R² = 0.035, p = 0.683) (Fig. 3). There were different numbers of species exclusive of burned and unburned plots in each temporal scale (see Supplementary Material). Importantly, most species exclusively found in burned areas were infrequent, with the exception of Hypoponera sp. 2, found in six burned plots, but only in the mid-term. The flood, inferred by the elevation, did not influence ant species composition at the three temporal scales (short, medium and long-term) (R² = 0.125, p = 0.234; R² = 0.034, p = 0.557; R² = 0.179, p = 0.442, respectively).
DiscussionThis study is the first, to our knowledge, to evaluate the relationship between both fire and flood disturbances in ant communities. Contrary to what we expected, we did not find a synergistic effect between fire and flood. Instead, ten days after a fire, we observed a dominant effect of fire disturbance, increasing the number of ant species in recently burned areas and not showing interactions with flood duration. Rather, the combined effect of fire associated with flooding was equal to the effect of fire alone (dominant factor). While the effects of dominant disturbances over many organisms are usually of long-term (e.g., Bruland et al., 1991; Vadeboncoeur, 2010), we found that the dominant effect of acute disturbance (fires) over chronic disturbance (floods) was present in the short and medium-terms, but disappeared with time. This suggests the resilience of the ant communities to this acute disturbance (fire).
We observed a fast increase in species richness immediately after the fires, a common response of many organisms, including ants (Andersen, 2018). This species richness increase soon after fire was accompanied by a marked difference in ant species composition between burned and unburned plots, with ten ant species found exclusively in the former ones. While fire can cause direct and indirect impacts on ant communities (Andersen, 2018; Arruda et al., 2020), the direct effects are often related to the death of colonies (Vasconcelos et al., 2017), which was probably not the case, due to the increase in ant species richness. Meanwhile, indirect effects are more likely to explain this rapid local increase in abundance and richness: ant colonies often increase their foraging activity after a fire due to habitat simplification and a decrease in ground-dwelling arthropod prey (Parr et al., 2007), which can be substantially affected by fires in the short-term (York, 1999). Therefore, the ground-dwelling ants need to allocate more workers to foraging activities after a fire and forage to longer distances (Sanders and Gordon, 2002). Notably, the higher ant richness was positively correlated with the frequency of occurrence, which was also higher in short-term burned areas.
The effect of fire on ant species composition and richness disappeared entirely after four years, indicating that although fire is a major disturbance in floodplains, its effects are temporary. Indeed, low-intensity fires do not seem to have many long-term effects on soil invertebrates, including ants and their prey (Andersen et al., 2005). After the recovery of the vegetation structure, including the grass-layer and the surrounding shrubs and small trees and consequently of soil and litter arthropods,’ the foraging activities of ground-dwelling ants may get back to pre-fire levels. Importantly, the Pantanal is a fire-prone ecosystem, and as such, it has many organisms adapted to fire events, at least to periodical and low-intensity ones (Manrique-Pineda et al., 2021). However, under increased fire frequency in the Pantanal, even fire-adapted organisms may face severe consequences (Pivello et al., 2021). Our results have implications for monitoring Pantanal biodiversity: ant communities can capture the signal of fires regardless of flood exposure, but only in the short and medium-term. Moreover, if the study purpose is not related to the effect of fire on ant communities, our data indicates that the interval of four years after a fire disturbance appears to be sufficient to disconsider its effect.
Contrary to our expectations, we did not detect any effect of the flood period on species richness and composition between the higher or lower elevation areas. This suggests that the species found in flooded areas are not a random subgroup of common species in adjacent non-flooded savannas and forest areas. Instead, the Pantanal wetland ant communities seemingly consist of ants with a high ability to colonize habitats that become seasonally available, including upper areas, like forest patches (Ribas and Schoereder, 2007) and even trees (Soares et al., 2013). Importantly, the Pantanal plains have been subject to flooding for millions of years (Assine and Soares, 2004), and it is known that this environmental filter has shaped its habitat structure and plant species distribution (da Silva et al., 2020). Therefore, ant resistance to floods can be interpreted as a result of their adaptations to a chronic and cyclical disturbance (Orians, 1975), which can act as important species filters (Drapeau et al., 2016), demonstrating that ants can adapt to disturbances (Burns et al., 2020).
Although many studies concluded that disturbances are important for community structuring, few have evaluated how chronic and acute disturbances can interact to structure them at multiple time scales. This gap can be explained by the complexity of experiments with this approach (Kéfi et al., 2019). For example, the unplanned fire events have started in neighboring farms, and were spatially determined in a large spot that hit nearby points. Thus, the spatial correlation at the local scale of the interest factors is common, as we detected in the model with the short-term fire effect. Indeed, as this is a natural experiment (sensuGotelli & Ellison, 2004), techniques to explain the spatial autocorrelation would also capture the fire effect since they are inseparable. Despite these potential limitations, since wetlands such as the Pantanal are facing increasing anthropogenic disturbances, studies on the effects of synergistic disturbances in such endangered habitats are timely. However, wetlands such as the Pantanal are facing increasing anthropogenic disturbances, and studies on the effects of synergistic disturbances in such endangered habitats are timely, despite potential limitations. The observed resilience of the Pantanal ant communities can have important implications since some of their ecological functions, such as nutrient cycling, might also be resilient to fire perturbations. Nevertheless, the studied ant community took some years to recover, and, thus, the increasing frequency of human-induced fire in the Brazilian Pantanal (Pivello et al., 2021) may impose a severe threat to ant diversity and their roles.
Conflict of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
FVA thanks CAPES (financing code-001) which provided his Ph.D scholarship and CNPq by PCI-MCTIC/MPEG (302198/2020-2). FC thanks CAPES for his post-doc scholarship. TJI and FBT were supported by CNPq (grants 309552/2018-4 and 306912/2018-0, respectively). REV thanks FAPEMAT (0602346/2017) and CNPq (313839/2019-0) to Desenvolvimento Científico Regional (DCR - 003/2016) support. We are highly in debt with Carol Peretz, for revising the English writing. We also thank Micael Rosa Parreira, Werther Pereira Ramalho, Jonas Maravalhas, Paulo De Marco, Rony Almeida and Frederico Neves, for helping with the revision of manuscript. The authors are grateful to Mr. Caio Pio (in memorian) and staff for their permission and support on the farm where the study was carried out.