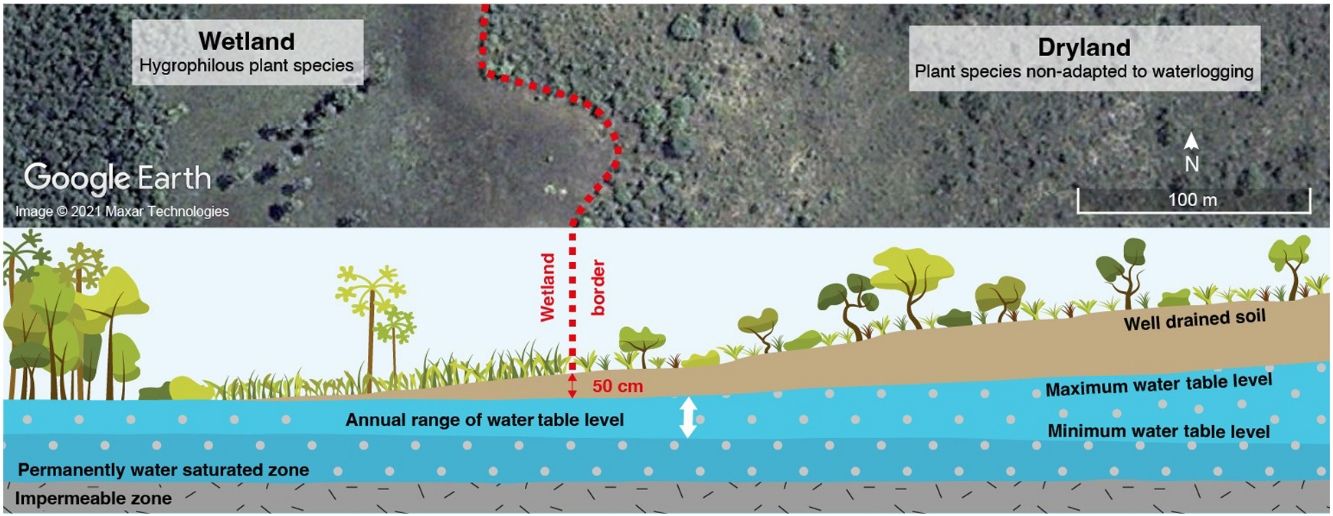
Wetlands are ecosystems at the interface between terrestrial and aquatic environments, subject to flooding by shallow waters or with temporarily to permanently waterlogged soils and specialized biota. Despite their great importance at global and local scales, these ecosystems have not been effectively protected in Brazil. The Cerrado wetlands are particularly neglected and misunderstood due to their distinctive hydrological functioning, imprecise maps, and multiplicity of vegetation types (e.g., wet grassland, vereda, palm grove, wet forest, gallery forest) that form complex and dynamic mosaics. Regional denominations of these wetlands often have subtle differences or redundancy, hindering their objective differentiation even by specialists. Regardless of vegetation differences, however, all Cerrado wetlands store and filter excess rainwater from the entire watershed, releasing it throughout the year to feed perennial streams and, ultimately, all of the main Brazilian rivers. Therefore, we argue that: (i) all wetlands in the Cerrado shall be unified in the legend of official large-scale maps that support environmental legislation; (ii) all vegetation types in Cerrado wetlands shall receive a unified treatment in the legislation, aligned with other wetlands in Brazil; (iii) the delimitation of wetlands for law compliance must be done on site, using objective indicators (hydrological, edaphic and botanical). We propose that all areas where the maximum elevation of the water table is less than 50 cm deep shall be mapped and protected as wetlands. That is the critical factor determining the hydromorphic soils and the specialized flora that differentiates these wetlands from the surrounding vegetation types in the Cerrado.
“Wetlands are ecosystems at the interface between aquatic and terrestrial environments; they may be continental or coastal, natural or artificial, permanently or periodically inundated by shallow water or consist of waterlogged soils. Their waters may be fresh, or highly or mildly saline. Wetlands are home to specific plant and animal communities adapted to their hydrological dynamics” (Junk et al., 2013). Wetlands, given their utmost importance for the provision of ecosystem services, have been considered as “the kidneys of the landscape” or “ecological supermarkets”, which places them as a global priority for conservation and restoration (Mitsch and Gosselink, 2011). Their degradation, thus, can lead to the collapse of entire ecological and socioeconomic systems. In addition, wetlands harbour high biodiversity, containing rare or threatened species unique to these environments (MEA, 2005). Numerous species of aquatic and terrestrial animals, including pollinating agents, depend on wetlands for food, shelter and/or reproduction (Gibbs, 2000).
In many countries, the concept of wetland is well understood, and their fragile ecosystems receive due attention, visibility, clear delimitation and legal protection (e.g., http://wetlands.org; https://www.nrcs.usda.gov/wps/portal/nrcs/detailfull/national/water/wetlands). The relevance of wetlands was consolidated worldwide with the Convention on Wetlands of International Importance, known as the Ramsar Convention, established in 1971 in the Iranian city of Ramsar (Matthews, 2013). Brazil is a signatory to the Ramsar Convention, which was incorporated into its legal framework in May 1996. In Brazil, however, the term wetland is rarely mentioned in environmental policies, legislation, academia and media, reflecting the lack of understanding of these relevant ecosystems. Wetlands have received different legal treatment and degrees of protection in Brazil depending on the vegetation types occupying them, and their treatment also varies between States and even between sectors within the same organization (Maltchik et al., 2018). Recent efforts have been devoted to categorizing wetland types in Brazil and demonstrating their importance and the urgent need for public policies aiming at their conservation (e.g., Junk et al., 2013; Cunha et al., 2015). However, the multiplicity of vegetation types occupying wetlands in Brazil and the incompleteness of the literature about wetland ecology and conservation require additional efforts to fill the gaps. As a consequence, the environmental laws also remain incomplete when referring to wetlands in Brazil (Grasel et al., 2019).
We here focus on the Cerrado wetlands because: (i) Cerrado is the largest among the Neotropical savannas, covering 22% of the Brazilian territory, and harbouring the headwaters of most watersheds in the country (Lima and Silva, 2007); (ii) recent land-use changes at a large scale in this biome have increased wetland degradation to a level unparalleled in other Brazilian biomes (Latrubesse et al., 2019); (iii) Cerrado wetlands are poorly understood relative to other wetlands in the country, such as the Pantanal or the Amazon River floodplain (Silva et al., 2000; Pott and Pott, 2004; Junk et al., 2018); and (iv) the existing legislation fails in clearly and adequately protecting all types of Cerrado wetlands (see Box 1 for the legal aspects and Table 1 for the vegetation types).
Box 1– The legal protection of Cerrado wetlands
The Native Vegetation Protection Law (#12.651, May 2012) did not manage to standardize and systematically treat wetlands in Brazil, which raises different interpretations and implementations by Brazilian states.
The law defines wetlands as “land surfaces periodically covered by water, originally covered by forests or other forms of vegetation adapted to flooding” (art. 3, item XXV). However, it does not establish clear rules for these areas. Some wetlands are treated as Permanent Preservation Areas (PPAs), such as mangroves (art.4º, VII), those around springs (art.4º, IV), or around veredas (art.4º, XI). Other wetlands are treated as Restricted Use Areas (RUAs), as is the case of “pantanais”, where “ecologically sustainable exploitation” is allowed (art.10). There is also the possibility that the Government may identify, on a case-by-case basis, wetlands that must be specially protected, declaring them PPAs (art.6, IX), which may require expropriation.
The Brazilian Superior Court of Justice (STJ) recently decided, applying the principle in dubio pro natura, that given their undeniable environmental importance, all wetlands must be understood as protected, either as PPAs or as RUAs, regardless of their nomenclature (Resp. 1787748/RS, Min. Rel. Herman Benjamin, DJe, IX.14.2020). This interpretation, however, is still far from being uniform in jurisprudence and administrative practice. The treatment of veredas, for example, remains very controversial. In some states, based on the literal interpretation of art.4, XI and in the absence of local rules, the conversion of veredas is allowed, as it is understood that the law protects only the marginal strip of 50 m from the permanently waterlogged space. In other states, either because there are more explicit rules or because they interpret the law in a more integrative way, both the veredas and the strip around them, which have a mere buffer function, are considered as PPAs. A change in the law has been proposed in the National Congress with the goal of formalizing the latter interpretation; this solution, however, would solve the problem of only one wetland type – the vereda –leaving several others (see Table 1) uncovered.
As we argue in this article, all wetlands have the same environmental importance, even though they harbour different vegetation types, so they should have the same degree of protection as RUAs, as decided by the STJ. In cases where they are outside PPAs, the wetlands must be included in the Legal Reserves required for each rural property. However, for these guidelines to be effective, identifying wetlands at a local scale is mandatory.
Vegetation types (phytophysiognomies) occupying wetlands in the Cerrado and their main attributes (based upon: Guarino and Walter, 2005; Ramos et al., 2006; Lima et al., 2015; Barbosa et al., 2019; and Nogueira et al., 2022).
| Formation | Vegetation types | Regional names | Vegetation description | Typical families, genera or species | Hydrological regime |
|---|---|---|---|---|---|
| Grassland | Wet grassland; Wet herbaceous grassland; Moist grassland | Campo limpo úmido; Campo úmido | Species-rich continuous ground cover of graminoids, forbs and subshrubs, without thick shrubs, treelets or trees. | Cyperaceae (e.g. Bulbostylis, Cyperus brasiliensis, Rhynchospora emaciata, R. robusta), Eriocaulaceae (Eriocaulon, Paepalanthus flaccidus, Syngonanthus nitens), Lentibulariaceae (Utricularia), Orchidaceae (Bletia catenulata, Cleistes, Habenaria), Poaceae (e.g. Andropogon virgatus, Paspalum lineare, Sacciolepis myuros, Sacharum asperum, Trichanthecium parvifolium), Polygalaceae (Polygala), Xyridaceae (Abolboda poarchon, Xyris jupicai, X. tortula). | Temporarily or seasonally waterlogged soil by groundwater rise (water table close to the surface). |
| Wet shrubby grassland; Wet herbaceous-shrubby grassland | Campo sujo úmido; Várzea (in part**); Brejo (in part) | Species-rich continuous ground cover of graminoids, forbs and subshrubs, scattered thick shrubs and treelets; trees rare or absent (canopy cover <5%). | Ground-layer flora very similar to the Wet grassland and part of the Palm swamp, differing by scattered shrubs (e.g. Chamaecrista, Duguetia furfuracea, Jacaranda caroba, Miconia albicans), treelets and trees (e.g. Casearia sylvestris, Ilex brasiliensis) from the surrounding vegetation, such as the Swamp gallery forest. | Temporarily or seasonally waterlogged soil by groundwater rise, or temporarily flooded from river or stream overflow. | |
| Earth mound grassland; Earth mounds; Termite savanna*** | Campo com murundus; Campo de murundus; Covoal; Monchão | Species-rich continuous ground cover of graminoids, forbs and subshrubs (the matrix) with spread conspicuous and well-drained earth mounds (murundus), generally associated with termites, with (or rarely without) treelets and trees on their top. | Two complementary floristically and structurally distinct components: i) the plains around the mounds with a herb-subshrub flora like the Wet herbaceous or shrubby grasslands; ii) the mounds, spatially restricted but conspicuous and well-drained, with treelets and trees (e.g. Alibertia edulis, Bowdichia virgilioides, Cecropia pachystachya, Curatella americana, Lafoensia pacari, Syagrus, Vochysia divergens, V. elliptica), and herb-shrub species (e.g. Bromeliaceae, Fabaceae and Poaceae) characteristic of dry savanna. | Poorly drained matrix temporarily or seasonally waterlogged or flooded by groundwater rise, but permanently well-drained mounds. | |
| Savanna | Palm swamp | Vereda; Várzea (in part)Brejo (in part) | Continuous layer of graminoids, forbs and subshrubs, with palm trees (12-15 m tall) scattered or clumped, and few tree and shrub species (canopy cover 5-10%). The species distribution associated with waterlogging severity results in different communities at the edge, the middle and the bottom. | Ground-layer composition very similar to Wet grassland and Wet shrubby grassland, differing by the presence of palms. The buriti Mauritia flexuosa is the most common, denser in the lower part, where waterlogging is more severe and shrubs more frequent; the less common and smaller palm tree buritirana Mauritiella armata occupies better-drained portions. Some shrubs are common (e.g. Ludwigia nervosa, Macairea radula, Miconia albicans, M. chamissois, Piper aduncum) and Swamp gallery forest trees may occur (e.g. Byrsonima umbellata, Calophyllum brasiliense, Cecropia pachystachya, Dendropanax cuneatus, Protium heptaphyllum, Richeria grandis, Tapirira guianensis, Xylopia emarginata). | Variation from temporarily or seasonally to permanently waterlogged soil by groundwater rise. |
| Mauritia palm grove; Mauritia palmland | Buritizal | Palm trees (8-15-m tall) in denser stands (canopy cover 10-50%) than in Palm swamp; scarce or frequently absent understory herbaceous and shrubby species due to Mauritia huge leaves and flower stalks. | Fully dominated by Mauritia flexuosa palm tree, although some trees (e.g. Calophyllum brasiliense, Cecropia pachystachya, Dendropanax cuneatus, Protium heptaphyllum, Xylopia emarginata), or treelet and shrub species (e.g. Jacaranda caroba, Miconia chamissois) can occur in low density compared with Palm swamp. | Temporarily or permanently waterlogged soil by groundwater rise and poor drainage. | |
| Forest | Swamp gallery forest | Floresta higrófila; Mata de galeria inundável; Mata de brejo; Mata alagada | Evergreen forest (canopy cover > 70%) generally associated with water bodies, with shrubby-herbaceous understory, usually surrounded by Wet grasslands or Wet shrubby grasslands. | Canopy dominated by trees like Calophyllum brasiliense, Dendropanax cuneatus, Ferdinandusa speciosa, Guarea macrophylla, Hedyosmum brasiliense, Richeria grandis, Protium spruceanum, Virola, Xylopia emarginata. Treelets and shrubs (e.g. Miconia, Piper) often occur at the edges or in the understory. Fabaceae are rare or absent. | Permanently waterlogged due to shallow water table, these forests can be seasonally flooded by streams and rivers overflow. |
| Floodplain forest | Ipuca; Impuca | Forest patch (canopy cover > 50%) in a discrete seasonally flooded depression (0.40 to 1.20 m below the surrounding grassy plain), not connected to watercourses. Understory absent, except at the edges. Exclusive to the middle plains of the great Araguaia River basin, in the central Brazilian states of Tocantins and Mato Grosso. | The most frequent tree species are Calophyllum brasiliense, Leptobalanus parvifolius, Tachigali vulgaris, and Vochysia divergens. | Seasonally flooded by groundwater rise and rain water. The grassy plain around is periodically flooded due to poor drainage and river overflow. |
*Grasslands are dominated by herbaceous plant species, with trees absent or very sparse; Savannas have trees spread over a herb-shrub stratum; and Forests are dominated by tree species.
Forests in Cerrado wetlands are not adequately treated by environmental laws, being only partially protected by a narrow strip of Permanent Preservation Area (PPA) around springs or along the banks of watercourses. Because the width of this strip is measured from the stream margin in the dry season (the “smaller width”, according to Art. 4º (I), Law 12,651, May 2012), extensive areas of seasonally or permanently waterlogged forests remain outside the PPA. In addition, isolated patches of wetlands which are not associated with water bodies are entirely unprotected. Equally inadequate is the legal delimitation of PPA around veredas, based on the horizontal distance starting from the permanently waterlogged area. In fact, the narrow strip of PPA includes only a small part of the seasonally waterlogged portions of a vereda, which can often extend far beyond the PPA and should not be separately treated or disregarded. Grassy wetlands located on the margins of water bodies in the Cerrado are often misinterpreted as “deforested land” by environmental agencies and professionals working on prosecution and adjudication of environmental offenses. These wetlands are then considered as environmental liability, and the landowners are inadvertently obliged to plant tree species where they never existed, under the equivocal pretence of “riparian forest restoration”. Afforestation in wetlands causes heavy losses in the rich herbaceous-shrubby flora and associated fauna, along with hydrological impacts.
Among the primary ecosystem services provided by wetlands (MEA, 2005), two are especially noteworthy in the Cerrado: (i) they function as filters and freshwater reservoirs, directly feeding most first-order streams in 8 of the 12 large Brazilian hydrographic regions (Lima and Silva, 2007), ensuring water quality and perennial rivers in the dry season; and (ii) store impressive amounts of carbon in organic soils (exceeding 200 Mg C ha−1) (Wantzen et al., 2012; França and Paiva, 2015). Furthermore, these ecosystems play a fundamental role in the local socio-economy, serving as a livelihood for several traditional human communities (Schmidt et al., 2011; Junk et al., 2013; Borges et al., 2016). Despite their undeniable importance for biodiversity conservation and provisioning of ecosystem services, the critical reality is that, without proper legal protection, Cerrado wetlands remain vulnerable and can be freely converted (drained or not) to any land use, no matter how disastrous this may be (Moreira et al., 2015; Pott et al., 2019; Brasil et al., 2021). Examples of these uses have been sand mining, crops, forestry with exotics (Pinus and Eucalyptus), pastures with African grasses adapted to waterlogged soils (e.g., Urochloa humidicola) and even urbanization. Impounding water or digging wells for livestock watering, irrigation, fish farming, landscape design and recreational purposes can cause the direct loss of wetlands and compromise their natural hydrological pulses.
The different vegetation types of Cerrado wetlandsDifferent vegetation types occupy wetlands within the Cerrado biome, ranging from forests to grasslands (main types described in Table 1, shown in Fig. 1), often forming complex landscape mosaics. The differences pointed out by phytogeographers, botanists and ecologists between vegetation types occupying Cerrado wetlands result in a broad number of concepts and denominations, not easy to recognize in loco even by specialists, which hampers the interpretation of the current law provisioning. In addition, transitional gradients exist within the wetland mosaics, and the whole mosaics are dynamic over time. The many terms used to refer to herbaceous vegetation include campo úmido, campo alagado, campo com/de murundus, várzea, brejo, and those referring to woody vegetation include vereda, buritizal, palmeiral, floresta higrófila, floresta pantanosa, mata de brejo, mata de galeria inundável, floresta alagável, mata paludosa, mata úmida or mata alagada. These terms often describe systems with only subtle differences, sometimes mixtures of concepts or also mere synonymy, with much redundancy and minor conflicts.
Main vegetation types occupying wetlands in the Cerrado (see also Table 1 for detailed information).
Hydrological functioning is the most critical factor determining wetland characteristics and is the principal descriptor when differentiating wetland types (Junk et al., 2013). Vegetation structure and composition within wetlands are strongly dependent on hydrological processes. The water that characterizes most wetlands in the Cerrado comes from the shallow or superficial water table, in contrast to wetlands in Pantanal or floodplain forests in the Amazon, where flooding results from rivers’ overflow during the rainy season (Silva et al., 2000; Pott and Pott, 2004; Junk et al., 2018; Cunha et al., 2015). Generally, as they are not subject to surface flooding and sediment input from river waters, the Cerrado wetlands have highly stable substrates.
The water table of a Cerrado wetland is fed by rainwater infiltration across the entire watershed. Surface runoff is rare in the Cerrado, restricted to hilly relief or drainage impediment; it is almost nonexistent in the flat areas with sandy and deep soils that comprise the majority of Cerrado land. Part of the rain falling on the vegetation evaporates before reaching the ground, but most infiltrates into the soil; the infiltrated water that exceeds the uptake by plant roots slowly percolates and accumulates at the bottom of the valleys. Because Cerrado rainfall is characterized by a marked seasonality, the hydrological pulses conditioning wetlands are also seasonal and predictable. The maximum elevation of the water table and annual variation in water table depth vary considerably within and between sites (due to edaphic and topographic factors), and between years (due to changes in rain volume and distribution).
Given the slow movement of infiltrated rainwater (Swarowsky et al., 2011), the maximum water table elevation in the Cerrado is usually recorded between April and May, when rainfall has already decreased (Manzione, 2018). Conversely, the lowest levels of the water table occur at the beginning of the rainy season (October–November). Plants quickly use the first rains and, only after this demand is met, the surplus recharges the water table (Oliveira et al., 2017). Part of this water goes further down, feeding deep aquifers. The remainder feeds surface water sources, which, due to these recharge processes, are perennial, in contrast to other extensive savannas on the planet (Tooth, 2000). In some Cerrado wetlands, impediment layers (rocks, laterite, soil textural differentiation) hamper drainage, restricting rain infiltration to deep reserves and, proportionally, increasing the recharge of surface water bodies. Wetlands resulting from impediment layers can occur even in plateaus or slopes, in regions where the geomorphology allows it.
Conditioning factors of plant communities of Cerrado wetlandsDespite differences in texture (from sandy to clayish) and genesis (sedimentary, residual or organic), wetland soils in the Cerrado are generally very acidic, with poor nutrient availability and high contents of organic matter. They are all subject to water saturation, and thus anaerobic conditions, at least temporarily. The accumulation and slow decomposition of organic matter resulting from permanent water saturation form dark organic soils which may exceed 1 m in depth, often forming peatlands, with impressive carbon storage (Wantzen et al., 2012; França and Paiva, 2015). Such edaphic conditions result in highly specialized flora (Justin and Armstrong, 1987; Rossatto et al., 2012; Villalobos-Vega et al., 2014; Silva et al., 2017).
Although some types of Cerrado wetlands are permanently waterlogged, in many cases plants need to be adapted to hyperseasonality, due to a large water table fluctuation over the year. There is water saturation especially at the end of the rainy season in such ecosystems, but the soil remains extremely dry and the water table very deep over long periods, making it particularly difficult to recognize these seasonal wetland types (Junk et al., 2013). The larger the variation within a wetland in the range of water table fluctuation and level of waterlogging, the higher will be its floristic diversity. These factors generally differ between the edges and the bottom of Cerrado wetlands, leading to associated floristic gradients (Meirelles et al., 2002; Oliveira et al., 2009).
The maximum water table elevation (minimum water table depth) is the main factor limiting the colonization of Cerrado wetlands by species from surrounding dry areas (Ribeiro et al., 2021). In all areas where the water table depth is less than 50 cm, at least during the period of its maximum elevation, sites will support specialized wetland flora and species sensitive to waterlogging will be unable to colonize (Pilon, 2016; Ribeiro, 2020). If this rhythm of annual elevation is changed by natural or anthropogenic factors, the limits of the wetland ecosystem also change, as discussed ahead.
Of the nearly 12,600 plant species in the Cerrado, around 20% are associated with vegetation types characteristic of wetlands (Mendonça et al., 2008). There are entire families whose species occur predominantly or exclusively in wetlands, slightly differing between vegetation types (examples in Table 1 and Fig. 2). These species are often considered indicators of these environments. Such specialization results in high degrees of endemism and many rare and endangered species (e.g. Eriocaulon burchellii, Microlicia ordinata, Mesosetum alatum, Polygala bevilacquai, Xyris goyazensis, X. paradisiaca) (Martinelli et al., 2014).
The absence or rarity of taxa that are otherwise abundant and species-rich in well-drained areas of Cerrado, such as Fabaceae (Leguminosae), also contributes to the floristic distinctiveness of wetlands in this biome. Also notable is the absence of some invasive exotic grasses (e.g., Urochloa decumbens, U. brizantha), which do not survive in waterlogged terrain.
Cerrado wetlands: a history of threats, negligence and ineffective conservation policiesThe ecosystem functioning in Cerrado wetlands is heavily dependent on natural hydrological pulses. Consequently, any factor that modifies the water table fluctuation regime will lead to ecosystem degradation, loss of diversity and ecosystem services, primarily by reducing soil carbon stocks and changing water yield. Wetland drainage directly modifies the hydrological regime with immediate impacts. As such, the conservation of wetlands and their functions depends on rigorously controlling changes in land use across the entire watershed, not just within the water-saturated zones.
Changes to vegetation can have large effects on runoff. Evidence from paired catchments shows that increases or decreases in aboveground biomass in more than 20% area of a watershed will cause, respectively, a decrease or increase in runoff (annual watershed flow) (Bosch and Hewlett, 1982; Brown et al., 2005). The greater the rain interception by tree canopies and the greater the transpiration, the less water will recharge the groundwater reserves, springs and rivers (Honda and Durigan, 2016). Therefore, silviculture, crop-forestry-livestock systems based on fast-growing tree species, and even high-density plantations of native trees where the former native vegetation was grassland or savanna will inevitably lower the water table in wetlands (Jackson et al., 2005; Ferraz et al., 2019). The opening and overexploitation of wells in the interfluvial zones, whether for crop irrigation or human supply, also lead to lowering the water table, putting its ecological functions at risk (Salem et al., 2017; Sun et al., 2019). Surface impermeabilization by infrastructure and urbanization, and inadequate management practices in agriculture and livestock lead to soil compaction, reducing infiltration and groundwater recharge and causing flow peaks and even floods during the rainy season (Honda and Durigan, 2017), likely resulting in environmental and economic damage. Unfortunately, the legal instruments which could prevent lowering the water table (e.g., Municipal Master Plan or Ecological-Economic Zoning) have not been effective.
Indirect damages to ecosystems resulting from the lowering of the water table in wetlands are rarely perceived. For instance, under natural conditions in grassy wetlands, a fire event eliminates only aboveground biomass (Schmidt et al., 2017) that immediately recovers by regrowth, followed by intense flowering (Araújo et al., 2013). If, however, the fire was preceded by lowering the water table, then extreme weather conditions can allow accumulated organic matter to burn for months (Maillard et al., 2009), severely compromising the ecosystem resilience. This degradation process is aggravated because burning organic soils results in high methane emissions (Turetsky et al., 2011), a greenhouse gas that is more harmful than CO2 normally emitted by burning aboveground biomass. Lowering the water table can also trigger biological invasions. Formerly waterlogged areas are prone to colonization by aggressive grasses which are widely disseminated in the Cerrado as weeds or invaders (e.g., Andropogon gayanus, Hyparrhenia rufa, Melinis minutiflora, M. repens, Urochloa brizantha, U. decumbens), as these species do not tolerate high water saturation (Tannus and Assis, 2004; Oliveira et al., 2009). Another example of a negative consequence of lowering the water table is increased colonization of grassy wetlands by native shrub and tree species adapted to well-drained environments, an example of woody encroachment (Giotto, 2015; Gonçalves et al., 2021). Despite leading to remarkable transformations in plant communities and the broader landscape, this increase in woody vegetation has been misinterpreted by many as an ecosystem “improvement”. Naturally, all changes mentioned also impact the native fauna inhabiting or using the resources provided by wetlands in the Cerrado (Stanton et al., 2018).
This array of threats, which is now well delineated, stems from the history of wetlands in the Cerrado, which have been the target of predatory and environmentally unsustainable public policies in recent decades. For example, the Provárzeas program (National Program for the Use of Irrigable Floodlands) was developed in the 1970s and 1980s and launched nationally in 1981 (Decree 86,146) to stimulate agricultural production in flooded lands, despite violating the guidelines of the Forest Code of 1965. To this end, the program encouraged the landowners, with financial, technical and administrative support from the government, to drain and cultivate floodplains on their land. The first initiatives in the Cerrado were implemented in Minas Gerais state, and what was understood as “floodplains” were precisely the Cerrado wetlands described here, particularly the grassy veredas and campos úmidos. Such ecosystems, in contrast to true floodplains fed by rivers, are not subject to fertilization pulses by annual floods, and their highly acidic, nutrient-poor and erodible soils are unsuitable for cultivation. Catastrophic occupation of wetlands has expanded to other Cerrado regions, widely replicating the officially stimulated degradation of these fragile ecosystems and severely hampering their biodiversity and ecosystem services.
Brazil has been a Ramsar Convention signatory for a quarter of a century, but this has not contributed to clarifying and disseminating the concept of wetlands, nor to value them in the Cerrado, a biome known as the “cradle of water” or the “water tank of Brazil”. Of the 27 official Ramsar sites in the country, only two (Parque Nacional do Araguaia/TO and APA Carste de Lagoa Santa/MG) are in the Cerrado biome, and both are located on its borders (MMA, 2021), with no sites recognized in the core area of the biome. Decision makers and people in general do not perceive that campos com murundus, campos úmidos with or without buritis, or even matas de brejo, among the many denominations presented here Table 1), fit perfectly into the internationally accepted concept of wetlands and should not be treated separately by law or by conservation policies. These vegetation types occupy a large area of the Cerrado and are rarely mapped when they do not have tree cover. In the Federal District of Brazil, wetlands were estimated to cover 3.4% of the territory (França et al., 2008). At the Serra Geral do Tocantins Ecological Station, they correspond to 4.8% (Cristo et al., 2016). Similar proportions are likely common within the whole Cerrado biome. Bozelli et al. (2018) estimated that wetlands occupy about 20% of Brazil. Although the Ramsar Convention mandated that signatory countries map their wetlands, this has not yet been achieved in Brazil, especially for small patches (Bozelli et al., 2018) such as those that make up the majority of wetlands in the Cerrado.
Concerning the legal framework, it is crucial to clearly define wetlands and facilitate their identification and delimitation in rural properties and urban areas. It must be recognized that the Native Vegetation Protection Law (12,651/2012) attempted to protect wetlands. However, the law’s application became imprecise, incomplete, and ineffective by poorly defining wetlands, treating the different vegetation types with distinct rules or simply ignoring some of them.
The fact that wetlands in Brazil are not mapped by governmental organizations (e.g. Environmental Ministry or state Secretaries), except when they occupy large areas, aggravates their vulnerability. That is even worse in the Cerrado, where much of the native vegetation in wetlands lacks tree cover. Surveys that map the remaining natural Cerrado vegetation (e.g., Sano et al., 2010, Beuchle et al., 2015) do not detail wetlands and rarely map vegetation types with less than 50% tree cover. Thus, the losses of Cerrado vegetation types with naturally low tree cover, particularly wetlands, are not even quantified. A recent study quantified 25% of palm swamps in the Cerrado as degraded over 34 years (Brasil et al., 2021). The inexistence of maps that allow identifying non-forest wetlands, and even open grasslands and savannas in interfluvial zones, has facilitated degradation and hampered legal inspection and possible penalties. This situation is improving, as the MapBiomas platform (2020) recently made available maps of Cerrado wetlands, grouped in a single legend class – ID 11: Wetlands. Such maps can be the basis for unified public policies to avoid land conversion and prevent land degradation of all wetland types within the Cerrado. That includes revising the provisions of the Native Vegetation Protection Law and aligning it with State laws.
Recommendations to enable the conservation of wetlands in the CerradoThe protection of wetlands requires action in several domains, among which we highlight the need for adequate legal framework and mapping with objective criteria. In addition, we present recommendations related to public policies, planning, communication, technical assistance, training, sustainable management and restoration.
(a) Identification and mapping at a local scale using objective criteria: flora, soils and depth of the water table
Regardless of the type of native vegetation, the recognition of a wetland ought to be based on the distinctive flora (species, genera or families of indicator plants), on hydromorphic soils, and the highest elevation (minimum depth) of the water table over the year. In remote sensing images, wetlands stand out from adjacent dry areas due to their different colour provided by the specialized flora and the organic and moist soils. Although these characteristics make it easy to map even small areas, the exact delimitation of wet, marshy or waterlogged space can only be carried out on site. Considering that the specialized flora occupies areas where the depth of the water table at the end of the rainy season (usually April–May in the Cerrado) is less than 50 cm (Giotto, 2015; Pilon, 2016; Ribeiro 2020), we propose that the delimitation of the wetland in loco is made at that period, adopting this depth as a criterion. Areas where the water table rises above 50 cm in depth at any time of the year cannot be converted for other uses. Without interventions that artificially lower the water table, these areas are not suitable for cultivation given the waterlogged soils which most cultivated plants cannot survive. According to the Natural Resources Conservation Service (USDA-SCS, 1972), the classic recommendation adopted in different regions of the world, and Brazil, is that soils should not be cultivated where the water table can rise above 60 cm depth, even if this elevation is seasonal. Therefore, delimiting the Cerrado wetlands based on a depth of 50 cm at the peak of the water table rise would not imply a loss of productive area.
In addition to the general maps delimiting wetlands as a whole for law compliance verification, maps at detailed scale that differentiate vegetation types and hydrological functioning (see Table 1, Fig. 1) are crucial to driving restoration planning and sustainable use practices. At the state and federal levels, the Brazilian agricultural research and development agencies should provide subsidies and instructions to restrict the use of wetlands for agricultural purposes, along with offering alternatives of ecologically sustainable use practices compatible with each wetland type and property size.
(b) Including unprotected wetlands in Legal Reserves
Although we argue that all Cerrado wetlands should be under the same legal status, this unification requires changes in the text of the current law. While waiting for these changes, we indicated (Box 1) how the Cerrado wetlands can be protected under the current law. However, we also explained that this protection is incomplete, with many areas or wetland types remaining unprotected. If not protected as PPA, any wetland, with or without arboreal vegetation, must be a priority to be included into the Legal Reserve (LR) within the property or to compensate for other properties (servidão ambiental). This recommendation is already included as one of the criteria for delimiting LR (art.14 of federal law 12,651): Art. 14. The location of the LR within a rural property must take into account the following studies and criteria: (I) the hydrographic basin plan; (II) Ecological-Economic Zoning; (III) the formation of ecological corridors with other LRs, PPAs, Conservation Unities, or with any other legally protected area; (IV) the areas of highest importance for biodiversity conservation; and (V) the areas of critical environmental fragility.
Wetlands fit perfectly and especially in items III (they are generally connected to riparian corridors), IV (they have exceptional biodiversity and high endemism, with peculiar species that do not occur in dry areas), and V (they are critically fragile areas, highly susceptible to contamination of water resources, vulnerable to soil erosion and siltation, and risk of high methane emission in case of disturbances). These three criteria place wetlands as a top priority in the demarcation of LRs, if they are not recognized as PPA (see Box 1). If classified as Restricted Use Areas, all wetlands can still be part of LRs, and sustainable use is allowed, according to Law 12,651.
(c) Regulation of interventions that compromise hydrological functioning
Any intervention within wetlands or upstream should be duly regulated when they threaten the hydrological functioning. Thus, the damming of watercourses that may submerge the vegetation or modify the hydrological regime of wetlands (deepening pulses and elevating groundwater) should be allowed only in exceptional cases of public utility. New wetland drainage works must be strictly prohibited due to direct damage to water resources and methane emissions resulting from soil movement. Drainage can artificially lower the water table and, naturally, it is a decisive factor in the degradation of wetlands. The grant for drilling new wells to capture groundwater in the Cerrado, even in interfluvial zones, must consider whether it will compromise the water table level in the wetlands.
(d) Training and awareness campaigns
The existence of wetlands in the landscape, their characterization, functioning, and their vast importance and extreme fragility have not deserved proportional space in research, teaching, extension and even less in the media in Brazil. Especially neglected are wetlands whose natural vegetation is grassy, without trees, which are common in the Cerrado biome and occur in the Pantanal or Pampa biomes, often being mapped as an environmental liability (Paula, 2019). Therefore, it is necessary to disseminate the existence and extreme importance of wetlands with grassy vegetation, in particular, to avoid disastrous “forest restoration” interventions. The high diversity of plants and animals that inhabit these areas, containing endemic and threatened species, must be widely announced.
It is also necessary to clarify that grassy wetlands surrounding gallery forests should not be transformed into “firebreaks to protect the forest against fire”, because clearing of these grassy areas and building the roads around them are a primary cause of Cerrado wetland degradation (Brasil et al., 2021). These strips of bare soil around wetlands result in erosion, loss of diversity and culminate in a complete disaster for the gallery forest itself over the years. Grassland formations around forests in wetlands are evolutionarily adapted to fire and, if “protected”, they tend to succumb to woody encroachment (Ribeiro et al., 2021).
Environmental and technical assistance technicians should be trained to identify wetlands at the local scale to recommend their protection, either as Permanently Protected Areas or Restricted Use Areas and or LR.
(e) Definition, validation and regulation of ecologically sustainable exploitation alternatives
There is a lack of studies validating the ecological sustainability and economic viability of wetland exploitation aligned with the “wise use” concept adopted by the Ramsar Convention on Wetlands (MEA, 2005). Plant extraction, beekeeping and meliponiculture, among other low-impact activities, if well managed, can be compatible with the conservation of biodiversity and maintenance of ecosystem services. Different vegetation types of Cerrado wetlands provide different opportunities to be sustainably used. The Native Vegetation Protection Act has different rules for APP and RUA, and the legal provisions regarding sustainable exploitation of natural or restored ecosystems depend also on the size of the rural property, and that must be considered.
(f) Restriction to forestry within the watershed
Tree plantations, whether of exotic or native species, in areas previously occupied by grassland or savanna under seasonal climate considerably increase rain interception and water uptake, compromising the functioning of wetlands (Brown et al., 2005; Jackson et al., 2005). Therefore, forestry should be restricted to a maximum proportion of 20% of watersheds previously covered by grassland or by savanna that has at least one wetland. This recommendation should drive land-use planning in regions not yet occupied by plantation forestry, where the economic-ecological zoning or municipal master plans should incorporate this criterion. In integrated production systems based on tree intercropping with herbaceous plants (Crop-Livestock-Forestry System – ICLF or Agroforestry System – AF), the basal area of arboreal vegetation should not exceed 15 m2 ha-1. To avoid compromising the groundwater recharge (Honda and Durigan, 2016), this limit represents the boundary between savanna and forest within the Cerrado (Abreu et al., 2017).
(g) Prevention and control of erosion processes in the whole watershed
Land cultivation itself does not imply a violation of environmental laws, but, frequently, inadequate practices can trigger erosion processes throughout the basin, generating sediment and chemical products that will accumulate in the wetlands. No-till agriculture keeping litter on the ground should be encouraged to minimize erosive processes and increase rainwater infiltration. Governmental and technical assistance organizations must be aware of, understand and disclose the importance of wetlands to encourage appropriate practices and curb harmful ones.
(h) Prevention and control of biological invasions
The cultivation of exotic plant species tolerant to wet environments (e.g. Leucaena leucocephala, Pinus spp., Urochloa humidicola) should be avoided or suppressed within a minimum safe radius from the wetland edge to prevent invasion of the natural ecosystems. This recommendation also refers to the legal exceptions for small properties (Art. 61-A, §13, IV), where planting exotic species is allowed within PPAs. For Pinus species, for example, 250 m away from wetlands would be the minimum recommended (Durigan et al., 2020).
The addition of fertilizers on neighbouring lands favours the proliferation of invasive exotic grasses (Bustamante et al., 2012), in addition to the eutrophication of adjacent water bodies. This can cause, among other effects, invasive algal blooms and expansion of populations of native (e.g., Typha) and exotic (e.g. Hedychium coronarium, Hydrilla verticillata, Panicum repens, Urochloa arrecta) dominant macrophytes that negatively affect aquatic biota (Thomaz, 2002).
(i) Ecosystem restoration in Cerrado wetlands
Any restoration intervention needs to be based on the features of the pre-existing vegetation type – the reference ecosystem. Restoration planning, therefore, must consider the specialized flora and hydrological functioning of each wetland type presented in Table 1. The most common mistake in Cerrado wetlands has been planting trees where they did not previously exist. The rare studies on wetland restoration in Brazil have shown that, under preserved hydrological conditions, the natural regeneration potential of herbaceous vegetation is generally high (Pilon et al., 2019; Durigan et al., 2020). When exotic plants invade the area, however, active intervention is needed. Restoration thus should start with the eradication and control of invasive species, followed by monitoring to verify the regeneration potential of the native vegetation. Planting seedlings, clumps or seeds of locally adapted species is only recommended if the ecosystem has lost its capacity to naturally regenerate, and only if the invasives are controlled. In most cases of wetland degradation, the central problem is the modification of the hydrological regime, especially the lowering of the water table. The restoration of the distinctive vegetation cover of wetlands can only be carried out after correcting the causal factor of the hydrological change.
ConclusionsDifferent vegetation types cover the Cerrado wetlands, and all of them have a vital ecological function of critical importance, namely filtering and storing rainwater and continuously supplying surface water bodies. By protecting and regulating water supply, wetlands support a high diversity of plants, animals and microorganisms, and human populations and their economic activities. The specialized biota of wetlands does not occur in the surrounding well-drained environments, allowing for high endemism. In addition to the uniqueness of their functions and biodiversity, these pieces of land are all extremely fragile, requiring solid protection mechanisms.
Brazilian laws have never given adequate legal treatment to wetlands in the Cerrado or even in the country as a whole (Junk et al., 2013; Grasel et al., 2019), making decisions and actions for their protection difficult or even impeding them. It is unjustifiable that they are not all treated equally by environmental laws, as already recognized by the Brazilian Superior Court of Justice. The recent unification of the Cerrado wetlands in the maps of the MapBiomas platform strengthens and sheds light on this argument and will be extremely important for decisions and actions related to their conservation.
If the difficulty of delimiting wetlands on a local scale has been an obstacle to their protection, consolidating the criterion of water table depth less than 50 cm in its maximum elevation as a determining factor, associated with the presence of wetland-specific flora and hydromorphic soils, will facilitate the easy and objective on-site verification and, therefore, the identification and precise delineation of wetlands to be protected in the Cerrado.
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank Hugo de Souza Dias by supporting us in the definition of the critical water table depth to maintain wetlands, Rodney Haulien Oliveira Viana by providing pictures of Ipucas, Bruna H. Campos for help with figures, and Samuel W. Flake for kindly revising the manuscript. GD, RSO and AP were supported by CNPq (productivity grants #309709/2020-2, #312270/2017-8 and 309698/2021-3). FAPESP supported RSO (NERC-FAPESP #19/07773-1), NALP (post-doctoral fellowship #2020/09257-8), and GD (thematic project #2020/01378-0).