Habitat loss is among one of the main causes of biodiversity decline worldwide. Therefore, assessing different dimensions of diversity such taxonomic, phylogenetic, and functional is important for more effective conservation strategies. Also, important but scarce, is the comparison of different life-stages which can bring insights due to different time delay on species responses to anthropogenic changes. Herein we assessed the influence of landscape-scale forest cover loss on different diversity dimensions of adult and juvenile tree assemblages. Our results showed that richness, phylogenetic and functional diversity were highly correlated for both life-stages. Forest cover loss leads to a decline in species richness more sharply in juveniles than adults, but in general, it did not affect phylogenetic and functional diversity. The responses among life-stages differed only for richness and phylogenetic mean pairwise distance. The negative impacts of forest cover loss on richness were not mirrored by phylogenetic and functional diversity, although there are some differences among life-stages. Our findings suggest that for practical purposes species richness is a primary and effective biodiversity measure at the landscape-scale. Furthermore, the stronger effects on juvenile assemblages indicate recruitment limitation and an impoverished future plant community, highlighting the importance to include different life-stages into conservation actions.
It is undeniable that we are experiencing a massive extinction event that surpasses the five previous Earth’s history mass extinctions (Barnosky et al., 2011). The current wave of biodiversity loss is mainly occurring due to a synergy among processes such as habitat loss, habitat modification, and climate change (Barnosky et al., 2011; Carmona et al., 2021). Several studies have reported that habitat loss leads to a diversity decrease in many taxa, including vertebrates, insects, and plants (Pardini et al., 2010; Rigueira et al., 2013; Spiesman and Inouye, 2013). The effects go beyond the loss of species, they also include evolutionary history and ecological processes reducing the ability of forests to sustain themselves in the long term (Mcdowell et al., 2020). However, the majority of these studies focused on species richness (SR), and there is a reasonable consensus that species richness per se often brings limited information on evolutionary history and function (Swenson, 2011). The integration of phylogenetic and functional diversity provides additional insights about ecological processes and might contain valuable information regarding ecosystem function (Cadotte et al., 2011). Thus, more recently, it is becoming common to include phylogenetic and functional diversity to capture important biodiversity dimensions that are also undoubtedly relevant to understand plant community assembly in human modified-landscapes.
In terms of plant phylogenetic diversity (PD) the outcomes are divergent so far. Negative effects of anthropogenic disturbances on phylogenetic relationships, either by reducing diversity or altering the structure by increasing phylogenetic clusters were recorded (Andrade et al., 2015). Otherwise, some studies detected an increase in PD or a phylogenetic overdispersed structure in disturbed areas, largely due to the incorporation of non-native species (Liu, 2016). However, there is an increasing number of studies finding that PD can be maintained in several tropical landscapes including highly fragmented forests (Santo-Silva et al., 2018). For functional diversity (FD), studies have reported that anthropogenic disturbances can cause either no effects (Flynn et al., 2009), negative (Suárez-Castro et al., 2020; Zambrano et al., 2020) or positive effects (Döbert et al., 2017) on plant community functional responses.
However, there is a clear bias towards studies focusing on adult tree assemblages. In this context, adult trees might reflect the accumulated responses to historical changes because they are prone to remain in the landscape for a longer time (Rigueira et al., 2013). On the contrary, recently established individuals, such as seedlings and juveniles, exhibit a higher sensitivity to habitat loss due to recent disruptive effects in reproductive, dispersal, and establishment processes influenced by deforestation (Rigueira et al., 2013). Therefore, distinct responses to anthropogenic disturbances are expected among life-stage groups, which can lead to divergent responses to richness, evolutionary history and ecological functions (Ernoult et al., 2006). Anthropogenic disturbances, can change local microclimatic conditions such as solar radiation available in the forest understory (Reis et al., 2021), which influence the establishment and survival of juvenile plants (Cerqueira et al., 2021). The younger assemblages represent the future of the forest, and can provide more accurate information on the consequences of forest loss in the long term (Mcdowell et al., 2020). Thus, although this knowledge is necessary for conservation decisions, these studies are still scarce.
Here we contrast adult and juvenile tree assemblage’s responses to forest cover loss on taxonomic, phylogenetic, and functional diversity. We predicted negative effects of forest cover loss mainly in taxonomic and functional diversity due to the increasing evidence suggesting maintenance of adult tree phylogenetic diversity in anthropogenic landscapes (Santo-Silva et al., 2018). We also predict that the impacts will be higher on juvenile than adult assemblages, because recruiting processes are impaired in the study area as forest cover is lost (Benchimol et al., 2017).
MethodsStudy regionThis study was conducted in 20 Atlantic Forest remnants in southern Bahia state, Brazil. The study area is located between two rivers (Jequitinhonha and Contas), classified as wet forest, where forest remnants exhibit similar soil types, topography, and floristic composition (Thomas et al., 1998). The predominant soil type is yellow latosol and red–yellow argisol (Fonseca et al., 2021), and regional climate is broadly classified as Af type in the Koppen system. Mean annual temperature is 24 °C, and annual rainfall averages 1800–2000 mm (INMET, 2021), without a significant seasonal climatic variation (Thomas et al., 1998). The southern Bahia region harbors one of the most diverse flora worldwide, exhibiting high levels of plant endemism and one of the highest records of plant richness (Martini et al., 2007).
Forest remnants located in an area of 3500 km2 were mapped using satellite imagery (RapidEye 2009–2010; QuickBird and World View 2009–2011), and manually digitized the land cover features based on differences in color, texture, and shape, at a scale of 1:10,000. Patches were delimited as polygons, and classified according to different forest types following the typologies provided by IBGE (2006). Based on this map we identified 40 potential forest remnants and from those, we performed a stratified sampling and selected 20 forest remnants, covering the full range of forest cover at different landscape scales (see Rocha-Santos et al., 2017, for further details of remnants select, and Table S1 for remnants details).
Floristic surveysMethods for adult and juvenile surveys are detailed elsewhere (Benchimol et al., 2017), but a brief overview is given here. For adult assemblages, we randomly established five 4 × 25 m plots inside the remnant, with a minimum distance of 50 m among plots, avoiding edge areas. In each plot, we sampled all adult trees with diameter at breast height (DBH) ≥ 5 cm and measured all individual tree height and DBH. For juvenile assemblages, we installed five sub-plots of 2 × 25 m within the tree plot and sampled all juveniles <5 cm DBH. After plant identification, we excluded all shrubs and lianas, considering just arboreal individuals in the juvenile dataset. Identifications were performed by experienced botanists to the lowest possible taxonomic level, based on regional herbarium collections (CEPEC/CEPLAC, HUESC/UESC and ALCB/UFBA).
Functional traitsFor all species, we obtained the following functional traits: seed dispersal mode, pollination syndrome, fruit size, regeneration strategy and wood density (Table S2). All selected traits are relevant to plant functional roles as food sources, seed dispersal, pollination, recruitment, and carbon storage. We obtained the wood density of all tree species in several global databases, such as “Plant Trait Database”, “Botanical Information and Ecology Network”, “Neotropical Tree Communities database”, “Global wood density database” (Chave et al., 2009), and Brazilian tree books (Lorenzi, 2022). We included wood density at the species level for 71% of species, when it was unavailable, we used the mean wood density at the genus level (25%), considering only South American species, and only 4% of species information was obtained for family level. For 98% of the morpho-species that were identified to the genus level, we used the genus information for South American species. All information about the regeneration strategy and pollination syndrome was obtained from the literature (see Rocha-Santos et al., 2019). Direct information on dispersal mode and fruit size was obtained for 21.44% of the species, and the remaining from literature data (78.55%). Fruit diameter and wood density for juvenile assemblage indicate potential values (based on the literature) for the species when adult.
Phylogenetic and functional diversityWe built a regional time-calibrated molecular phylogenetic tree using the juvenile and tree assemblages recorded in the 20 forest remnants (Supporting information — Phylogenetic Data). Then, we calculated two phylogenetic metrics: Faith’s PD, and the phylogenetic Mean Pairwise Distance (pMPD). The pMPD measures the average phylogenetic distance among pairs of individuals drawn at random from a sample. Phylogenetic metrics were calculated using pd and mpd functions in the “picante” package.
We used an equivalent approach to evaluate the functional diversity using a functional dendrogram. We calculated the Petchey & Gaston FD (Petchey and Gaston, 2002) and the functional Mean Pairwise Distance (fMPD). To calculate FD we used the FD-dendro in the package “fundiv”, using a Gower’s dissimilarity matrix (Podani and Schmera, 2006). We calculated fMPD from the functional dendrogram using the mpd function in the “picante” package. We also obtained the community-level weighted mean (CWM) of trait values to explore which trait drive functional diversity. CWM was calculated for continuous variables (fruit diameter and wood density) as the mean value of the trait weighted by the relative abundance of each taxon (Garnier et al., 2004).
For both phylogenetic and functional diversity metrics, we calculated the standard effect sizes (SES), as the observed value — mean expected value/standard deviation of the expected value. Expected values were calculated from 1000 randomizations from the species pool keeping equal species richness. The SES values indicate to what extent communities are phylogenetically or functionally clustered (negative SES values) or dispersed (positive SES values) than expected by random. All phylogenetic and functional metrics were calculated for adult and juvenile assemblages separately. We reported pMPD in millions of years and SES is expressed in units of standard deviation. The SES values were calculated using the sespd function in the “picante” package.
Forest coverWe adopted a patch-landscape approach, in which the response variables were evaluated within the forest and the amount of forest cover was measured within a specific area around each sampling remnant (Tischendorf and Fahrig, 2000). The forest cover percentage was quantified from the center of each forest remnant at tree spatial scales (500 m, 1000 m and 1500 m). These scales were chosen based on the literature showing that tree structure (Rocha-Santos et al., 2016) and tree richness and abundance (Rocha-Santos et al., 2017) best respond at scales around 1000 m in the same landscapes. We considered only mature and secondary native forests and excluded plantations (such as cocoa, rubber, and eucalyptus) on forest cover amount calculation. Forest remnants were immersed in landscapes ranging from 98% to 3% of forest cover.
Statistical analysesFirst, we performed a Spearman correlation among all diversity dimensions separated by adult and juvenile assemblages. Then, we evaluated the best scale of effect by comparing the estimated values of the linear model between each response variable and the percentage of forest cover in landscapes of varying radii. The radius with the highest estimated value for each variable was established as the standard for all subsequent analysis (Table S3). We evaluated the spatial dependency of the residuals of linear models between each diversity dimension and forest cover loss at the best scale of effect by calculating the Moran’s index. In general, the models did not exhibit spatial dependency (the only exception was juvenile richness and adult fMPD — see Table S4). Finally, to evaluate the responses of the three diversity dimensions to forest cover loss we performed analysis of covariance (ANCOVA). We also performed analysis of covariance between CWM and forest cover, to assess the effects of forest cover on the specific functional traits. The life-stages (adult and juvenile) were considered a factor and forest cover as a covariate. We previously evaluated data normality and homogeneity assumptions. All analyses were carried out in R software.
ResultsWe recorded a total of 1950 adults from 507 species and 3397 juveniles from 441 species. After the exclusion of 51 species for which we did not find molecular information, we ended up with 1868 adults from 457 species and 52 families, and 3183 juveniles from 400 species and 54 families. The three dimensions of diversity (SR, PD and FD) were highly correlated, for both adults and juvenile assemblages (rs ≥ 0.7), thus we presented only SR results (Fig. S1 and S2). Phylogenetic metrics were in general not correlated with the equivalent functional metrics for both life-stage (PD × FD; pMPD × fMDP; SES PD × SES FD; SES pMPD × SES fMDP) (Figs. S1 and S2). For both adults and juveniles, FD was not correlated with the other functional metrics (SES FD, fMPD and SES fMPD), the same pattern was found for phylogenetic metrics (SES PD, pMPD and SES pMPD).
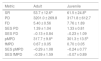
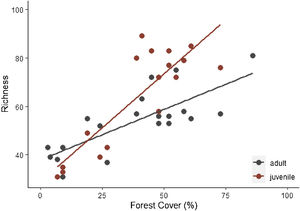
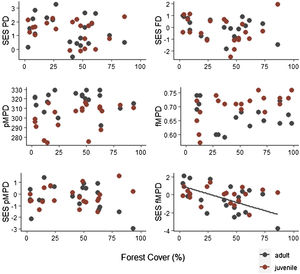
Specie richness (SR) differed between both ontogenetic stages that also showed an interaction with forest cover (Table 1). Both ontogenetic stages responded negatively to forest cover loss, with a stronger richness decrease for juvenile than adult assemblage (interaction term P < 0.001; adult slope: 0.42 and juvenile slope: 0.96; Fig. 1 and Table 1). In general, the phylogenetic and functional metrics were relatively similar between adult and juvenile assemblages (Table 2). Except for the mean phylogenetic distance (pMPD) that was higher in adult than juvenile assemblages, but neither ontogenetic stage responded to forest cover loss (Table 1 and Fig. 2). The average values of SES PD and SES pMPD were similar between adult and juvenile assemblages and did not respond to forest cover loss (Tables 1 and 2). Addressing functional metrics, mean functional distance (fMPD) was similar between ontogenetic stages and did not respond to forest cover, but juvenile and adult assemblages respond differently to forest cover loss (significant interaction, Table 1). Whereas, SES fMPD decreased as forest cover decreases at the landscape-scale, but only for adult assemblages (Table 1 and Fig. 2). Additionally, roughly 50% of the forest remnants presented a more clustered phylogenetic and functional structure than expected by chance (negative SES pMPD and SES fMPD values) in both life-stages (Fig. 2). Individual functional traits of wood density (CWM) were not affected by forest cover loss in both life stages (Table 3). However, the CWM for fruit diameter was different between adult and juvenile (Table 3). Also, they respond differently to forest cover variation (significant interaction, Table 3), with a greater decline in fruit diameter of species within the juvenile stage (adult slope: −0.003 and juvenile slope: −0.613).
Results of analysis of covariance (ANCOVA) showing the response of the diversity dimension for the two ontogenetic stages (adult and juvenile) to forest cover loss in 20 forest remnants from the Atlantic Forest, Bahia, Brazil.
| DF | MS | F | P | ||
|---|---|---|---|---|---|
| SR | FC | 1 | 0.416 | 4.553 | <0.001 |
| OS | 1 | −12.910 | −2.236 | 0.032 | |
| FC*OS | 1 | 0.546 | 4.231 | <0.001 | |
| Residuals | 36 | 9.619 | |||
| SES PD | FC | 1 | −0.010 | −1.134 | 0.264 |
| OS | 1 | −0.198 | −0.342 | 0.734 | |
| FC*OS | 1 | 0.004 | 0.289 | 0.774 | |
| Residuals | 36 | 0.960 | |||
| SES FD | FC | 1 | −0.013 | −1.367 | 0.180 |
| OS | 1 | −0.376 | −0.647 | 0.522 | |
| FC*OS | 1 | 0.007 | 0.550 | 0.585 | |
| Residuals | 36 | 0.968 | |||
| pMPD | FC | 1 | −0.040 | −0.405 | 0.688 |
| OS | 1 | −26.775 | −3.975 | <0.001 | |
| FC*OS | 1 | 0.259 | 1.846 | 0.073 | |
| Residuals | 36 | 11.650 | |||
| fMPD | FC | 1 | 0.000 | −0.490 | 0.627 |
| OS | 1 | −0.024 | −0.891 | 0.379 | |
| FC*OS | 1 | 0.001 | 2.354 | 0.024 | |
| Residuals | 36 | 0.043 | |||
| SES pMPD | FC | 1 | −0.014 | −1.683 | 0.101 |
| OS | 1 | −0.752 | −1.373 | 0.178 | |
| FC*OS | 1 | 0.020 | 1.735 | 0.091 | |
| Residuals | 36 | 0.946 | |||
| SES fMPD | FC | 1 | −0.038 | 0.011 | 0.001 |
| OS | 1 | −0.836 | 0.686 | 0.231 | |
| FC*OS | 1 | 0.030 | 0.015 | 0.056 | |
| Residuals | 36 | 1.143 |
FC = Forest cover, OS = ontogenetic stages, SR = species richness, SES PD = standard effect size of phylogenetic diversity, pMPD = phylogenetic mean pairwise distance, SES FD = standard effect size of functional diversity, fMPD = functional mean pairwise distance, SES pMPD = standard effect size of phylogenetic mean pairwise distance, SES fMPD = standard effect size of functional mean pairwise distance, DF: degrees of freedom; MS: mean square; F: F-statistic; P: P-value (significative <0.05).
Significant values (P < 0.005) are highlighted in bold.
Mean plant species richness, phylogenetic and functional metrics (±SD) among adult and juvenile assemblages in 20 forest remnants from the Atlantic Forest, Bahia, Brazil.
| Metric | Adult | Juvenile |
|---|---|---|
| SR | 53.7 ± 12.6a | 61.5 ± 24.8b |
| PD | 3201.0 ± 269.8 | 3171.8 ± 612.7 |
| FD | 5.40 ± 0.56 | 7.76 ± 1.89 |
| SES PD | 1.39 ± 1.04 | 1.33 ± 0.81 |
| SES FD | −0.13 ± 0.84 | −0.23 ± 1.09 |
| pMPD | 317.7 ± 9.8a | 301.3 ± 13.5b |
| fMPD | 0.67 ± 0.05 | 0.70 ± 0.05 |
| SES pMPD | −0.29 ± 1.08 | −0.24 ± 0.77 |
| SES fMPD | −0.39 ± 1.59 | −0.07 ± 0.89 |
Metrics with different letters for adults and juvenile showed a significant difference (P > 0.01), accordingly to ANCOVA results (Table 1).
SR = species richness, PD = phylogenetic diversity, FD = functional diversity, SES PD = standard effect size of phylogenetic diversity, SES FD = standard effect size of functional diversity, pMPD = phylogenetic mean pairwise distance, fMPD = functional mean pairwise distance, SES pMPD = standard effect size of phylogenetic mean pairwise distance, SES fMPD = standard effect size of functional mean pairwise distance.
Relationship between forest cover amount and phylogenetic and functional diversity metrics of adult and juvenile tree assemblages in 20 forest remnants from the Atlantic Forest, Bahia, Brazil. SES PD = standard effect size of phylogenetic diversity, SES FD = standard effect size of functional diversity, pMPD = phylogenetic mean pairwise distance, fMPD = functional mean pairwise distance, SES pMPD = standard effect size of phylogenetic mean pairwise distance, SES fMPD = standard effect size of functional mean pairwise distance.
Results of analysis of covariance (ANCOVA) for Community Weighted Means (CWM) of fruit diameter and wood density for the two ontogenetic stages (adult and juvenile) to forest cover loss in 20 forest remnants from the Atlantic Forest, Bahia, Brazil.
| DF | MS | F | P | ||
|---|---|---|---|---|---|
| Fruit diameter | FC | 1 | −0.003 | −0.782 | 0.439 |
| OS | 1 | −0.615 | −2.259 | 0.030 | |
| FC*OS | 1 | 0.012 | 2.540 | 0.016 | |
| Residuals | 36 | 0.438 | |||
| Wood density | FC | 1 | 0.025 | 1.179 | 0.246 |
| OS | 1 | −0.043 | −0.032 | 0.975 | |
| FC*OS | 1 | −0.025 | −0.818 | 0.419 | |
| Residuals | 36 | 2.272 |
DF: DEGREES of freedom; MS: mean square; F: F-statistic; P: p-value (significative <0.05).
Significant values (P < 0.005) are highlighted in bold.
Our results unveil differences in adult and juvenile tree responses to forest cover loss, which resulted in a marked decline in tree species richness in the Atlantic Forest, more sharply for juvenile than adult assemblages. Surprisingly, the phylogenetic and functional diversity, using metrics not correlated with species richness, were maintained along the gradient of forest cover for both ontogenetic stages. The only exception was the increase in SES fMPD with decreasing forest cover, but only for adult assemblage indicating over-dispersion of functional traits in deforested landscapes. We revealed that the evolutionary value of disturbed forests is high and possibly those forests are also partially maintaining ecosystem functions in present and future plant communities.
The pervasive effects of forest cover loss on plant species richness were expected and have been previously documented elsewhere (Benchimol et al., 2017; Montoya et al., 2008). The direct comparison of adults and juveniles showed distinct responses to landscape-scale forest cover loss with stronger decline in juvenile, probably due to the shorter life span of juvenile individuals. The drastic decrease in juvenile assemblage may be a reflection of demographic bottleneck effects occurring in some previous stages such as reproduction, dispersal and establishment. As previously shown in our landscapes, where forest cover loss impairs the regeneration process thus compromising the future tree community (Benchimol et al., 2017). We also showed that SR was a stronger predictor of PD and FD metrics (correlation ≥ 0.7), evidencing that these metrics are not adequate to measure the phylogenetic and functional diversity. Thus, we recommend using metrics standardized by effect size (SES) or Mean Pairwise Distance metrics. It is expected that in highly diverse regions, PD increases at a similar rate as SR, and thus, PD tends to be with SR (Fjeldså, 1994).
Conversely, phylogenetic diversity and structure (pMPD and SES pMPD) were maintained along the forest cover gradient. This result has been supported by previous studies on adult tree assemblages (Santo-Silva et al., 2018) and suggests that traits related to high susceptibility to disturbance are convergent or have a low phylogenetic signal. Some ecological traits that make species more susceptible to environmental changes, such as sensitivity to light incidence and drought tolerance, are not exclusive to phylogenetically related species (Niinemets and Valladares, 2006). Interestingly, the mean phylogenetic diversity was higher in adults than in juvenile assemblage and phylogenetic diversity and structure of juveniles were not affected by forest cover loss. Because juvenile assemblages might reflect current responses to anthropogenic disturbances we expected a greater phylogenetic impoverishment on this ontogenetic stage, as previously recorded for chronic disturbances (Ribeiro et al., 2016). On the contrary, our results revealed that there is a high variation in the values of phylogenetic diversity and structure between forest remnants, both for juveniles and adults. This variation is not influenced by forest cover loss, corroborating a recent study where plant phylogenetic diversity was little influenced by landscape conditions (De Pauw et al., 2021).
Regarding functional diversity, forest cover loss affected the SES fMPD only for adult assemblage. This indicates that after controlling for richness, an over-dispersion of functional traits is observed in deforested landscapes. Contrary to our expectation, this result shows that adult tree functional diversity is higher in species-poor fragments, with few species dispersed across a wider breadth of functions. Yet, functional divergence in tree assemblage has been previously reported (De Pauw et al., 2021; Döbert et al., 2017; Rocha-Santos et al., 2019). This pattern indicates that while tree assemblages selectively lose some functional traits due to forest cover loss, these traits are replaced by divergent traits favored by anthropogenic disturbances. Nonetheless, when the traits were analyzed individually, we show that forest cover did not influence wood density independent of the life-stage. However, forest cover affected fruit diameter, with the assemblage of juvenile species showing potentially smaller fruits than adults in more deforested landscapes.
It is important to highlight that trait selection can affect the functional metric results, therefore, we selected important traits that are related to some ecological processes (such as regeneration, dispersal, pollination, carbon stock). Also, a massive effort to collect trait data was performed, but we are still far from having a comprehensive coverage. Although we acknowledge that trait selection and its coverage could change part of the results reported here, we emphasize that this is an inherent problem of FD metrics. Indeed, although our forest fragments within highly forested landscapes were relatively well preserved, these forests suffered from disturbances such as selective logging and hunting, therefore they are unlikely considered as pristine. Thus, we suggest that additional studies with different traits and functions, and local levels of disturbance would be necessary to generalize to plant assemblages worldwide.
To conclude, our results reveal a high conservation value of disturbed forests. In terms of evolutionary history and functional trait maintenance, this is undoubtedly true. Although some diversity metrics differed between life-stages, they did not change as a function of forest cover loss, suggesting that extinction debt might not be masking long-term effects of deforestation on phylogenetic and functional diversity. However, forest cover loss in the southern Bahia has not only noticeable effects on tree assemblages, but also impacted on ecological processes such as fruiting, seed dispersal and herbivory (Benchimol et al., 2017; Dodonov et al., 2016; Hambuckers et al., 2020). These results combined can reinforce that the profound effects of deforestation already detected on richness are not mirrored by the phylogenetic and functional metrics evaluated here, regardless of life stage. Taxonomic diversity, due to its simplicity, was suggested as one effective index of biodiversity loss in anthropogenic landscapes, as thresholds in taxonomic diversity precede the loss of phylogenetic diversity (Boesing et al., 2018). In addition, we recommend using metrics not correlate with species richness to improve phylogenetic and functional conclusions. Thus, the noticeable conservation value of deforested landscapes found here in terms of function and phylogenetic diversity reinforces the need and importance to conserve every remnant of tropical forest. However, the combined approach integrating the different dimensions of biodiversity and different life-stages used here reinforce that increasing forest cover at the landscape scale is urgent to safe-guard tree taxonomic diversity.
This study is part of the ‘Rede Sisbiota’ project (publication Nº47), and it is funded by the Brazilian Council of Science and Technology (563216/2010-7), Coordination of Superior Level Staff Improvement (001), and Universidade Estadual de Santa Cruz–UESC (PROPP 073.6764.2020.0010464-02). We thank Francisco Sanches for providing juvenile data. We are grateful for helpful comments from Geomatics and Landscape Ecology Friday Discussion Group, from Carleton University.