Freshwater biodiversity is threatened at global scale, thus, understanding how it responds to anthropogenic interferences is critical, especially in regions where human disturbances have quickly altered natural ecosystems. Here, we address the effects of landscape structure in Brazilian Cerrado on freshwater community diversity of phytoplankton, periphyton, zooplankton and fish, and instream’s features (physicochemical and biological indicators of water quality, and water velocity), and the effects of instream’s features on freshwater community diversity. We analyzed the data at different spatial scales (50, 100, 150, and 200 m, and watershed level), using structural equation modeling. We found that percentage of native vegetation (%NV) at watershed level explained Cladocera’s abundance and Shannon-wiener with a negative relationship. Landscape compositional heterogeneity (SHDI) at 200 m explained Periphyton abundance with a positive relationship. %NV at 50 m explained dissolved oxygen with a positive relationship. Total coliforms explained Cladoceras’s abundance with a positive relationship. Conductivity explained Cladocera’s abundance and richness with a negative relationship. Our findings show that landscape changes are favoring some biological groups, which can lead to freshwater biotic homogenization. Thus, the unsustainable expansion of agriculture can compromise freshwater biodiversity and water quality in Cerrado.
Even though freshwater biodiversity crisis has received more attention in the last years (Arthington, 2021), guidelines to improve water quality with focus on freshwater ecosystems health are still rare (Bogardi et al., 2020). The management of freshwater resources has been designed to give more support to human water security than the integrity of natural ecosystems (Harrison et al., 2018). The structure of aquatic communities can be affected even by low rates of habitat loss (Dala-Corte et al., 2020), and the responses of freshwater biodiversity to environmental alterations can vary among taxonomic groups (Faquim et al., 2021), and spatial scales (Barbosa et al., 2019). In this way, understanding how freshwater biodiversity responds to anthropogenic interferences is urgent, especially in regions where human disturbances have quickly altered natural ecosystems.
The loss of natural vegetation surrounding watercourses can alter water discharge modifying several streams’ physical and physicochemical parameters (de Paula et al., 2022). Habitat loss can also reduce the presence of terrestrial invertebrates or the input of allochthonous items such as fruits, leaves, branches, trunks, which are refugia, reproductive sites and food for aquatic organisms (Lo et al., 2020). Light regime, temperature, pH, and nutrient availability are also changed with the reduction of riparian forests (de Paula et al., 2022), influencing the primary productivity (Fernández et al., 2022; Junk et al., 2022). In agricultural landscapes, soil surface runoff and the input of fertilizers and pesticides cause streams pollution and sedimentation impairing water quality (Latrubesse et al., 2019).
Different aquatic taxa have been suggested as reliable bioindicators of landscape disturbance (Camilo-Cotrim et al., 2022; Dala-Corte et al., 2020). However, the interaction between land cover changes at different spatial scales, the conditions of instreams habitat, and aquatic communities is complex (Leal et al., 2016). Thus, predicting biodiversity response across anthropic landscapes and identifying the landscape variables that most affect aquatic organisms and bioindicators for different spatial scales is a challenge.
The Brazilian Cerrado ecoregion is one of the world hotspots of biodiversity (Myers, 2000), comprising a large number of headwaters that supplies the principal watersheds of South America (Latrubesse et al., 2019), but has only 8% of the total territory under legal protection and is one of the most threatened Brazilian ecoregions (Latrubesse et al., 2019; Alencar et al., 2020). In the last decades, Cerrado has become one of the most important agribusiness regions in Brazil, and lost more than 50% of its total area in less than 60 years (Alencar et al., 2020). Few studies have explored the effects of terrestrial landscape structure to predict freshwater biodiversity in Cerrado using variables that exceed habitat amount, and at different landscape spatial scales (Barbosa et al., 2019; Dala-Corte et al., 2020; Machado et al., 2016). Also, the relationship between streams’ water quality and landscape changes is still poorly known. Physicochemical and biological parameters of freshwater ecosystems can be proxies of the health of rivers at watershed level, because these ecosystems are sinks of the landscape, due to surface run-off and exchanges with groundwater (Bogardi et al., 2020).
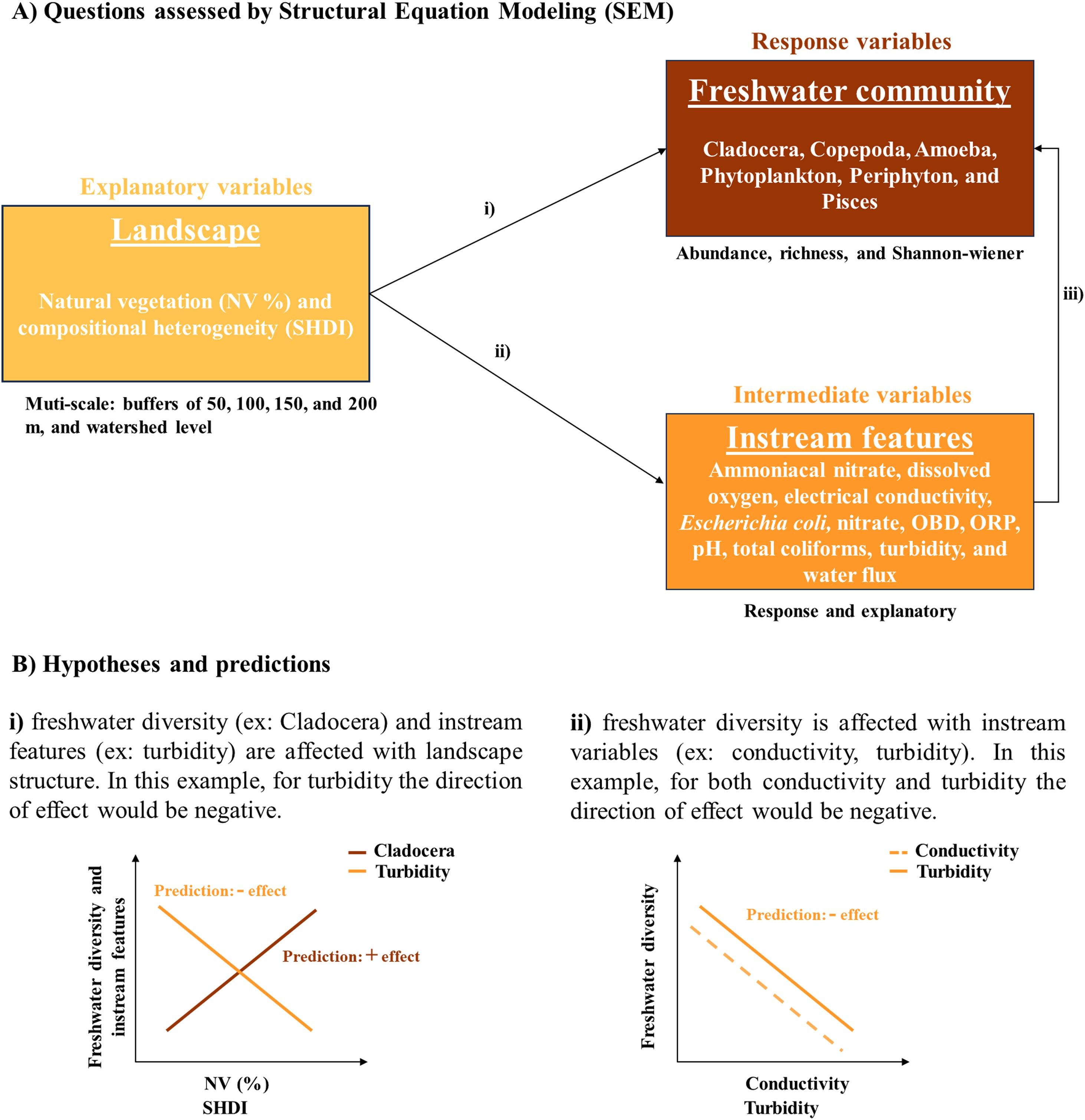
Here, we address the effect of terrestrial landscape structure at multi-scale (local and watershed levels) on freshwater community diversity (Fig. 1A; i) and on instream features (Fig. 1A; ii) in intensive farming landscapes within Brazilian Cerrado. Furthermore, we address the effects of instream features on freshwater community diversity (Fig. 1A; iii). Forested areas along streams contribute to the quality of stream’s habitats (de Paula et al., 2022, 2013), water quality and provision (Junk et al., 2022), and terrestrial and aquatic biodiversity (Brito et al., 2020). In the study area, landscapes with higher vegetation amount and compositional heterogeneity support higher diversity of diverse taxa (Santos et al., 2022; Martello et al., 2023; de Sousa et al., 2022; Silveira et al., 2023). Hence, we hypothesize that freshwater community diversity and instream features (Fig. 1B; i) are influenced by terrestrial landscape structure and that freshwater community diversity is also influenced by instream variables (Fig. 1B; ii).
Schematic representation of the questions addressed in the study (Ai, ii, and iii) using Structural Equation Models (SEM), and hypotheses and predictions (Bi and ii). Predictions: we expect that streams within landscapes with higher vegetation amount and compositional heterogeneity have higher freshwater community diversity (Bi), and suitable values of physicochemical and biological indicators of water quality and water velocity (Bi). Also, we expect that streams with suitable values of physicochemical and biological indicators of water quality and water velocity have higher freshwater community diversity (Bii). The direction of effect will vary according to each stream feature. pH, potential of Hydrogen; ORP, Oxidation Reduction Potential, and OBD, Oxygen Biological Demand.
Higher amounts of agroecosystems such as pasture and agriculture, and urban areas in watersheds tend to negatively influence the zooplanktonic community, mainly specialist species (Dos Santos et al., 2024). Intensive agriculture and livestock farming dominate our study area. Thus, we expect a greater effect of terrestrial landscape structure at large than fine spatial scales for this group. For fish, periphyton, and phytoplankton, we expect an effect of terrestrial landscape structure at fine spatial scales, because these organisms are more sensitive to changes in physical habitat structure (fish and periphyton) and shading (phytoplankton), which are directly influenced by changes in land cover closer to the streams (Barbosa et al., 2019). With this study, we hope to contribute to fill a knowledge gap in Cerrado, and provide some indicators of freshwater ecosystem health at local and watershed level.
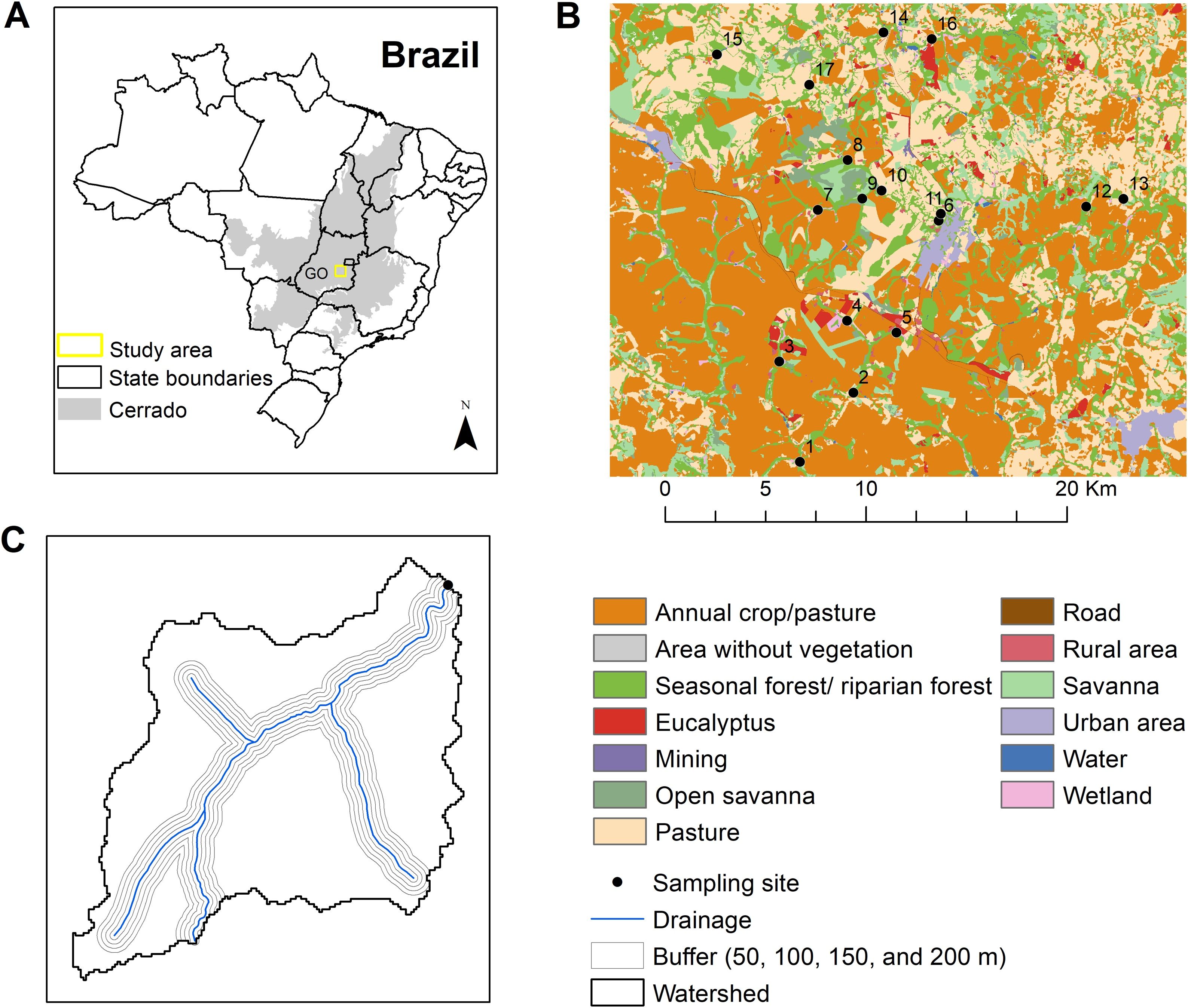
Material and methodsStudy area and community samplingWe studied watercourses in a landscape encompassing a Long-Term Ecological Research (LTER) network the LAND-LTER (Agricultural landscape dynamics and impacts on biodiversity) project, in Central-West Brazil (Santos et al., 2021; Fig. 2A). The landscape is placed in one of the most important Brazilian agribusiness regions dominated by intensive farming systems such as soybean, maize, and livestock (Santos et al., 2021).
Geographical distribution of sampling sites and land cover of the LAND-LTER landscape. (A) Limits of Brazil with the Cerrado ecoregion (dark gray polygon) and the study area (yellow square). GO is the Goiás state; (B) Land cover composition of the study area. Black circles are the 17 sampling sites. Different colors correspond to land cover classes according to the legend; (C) Multiscale approach used to calculate the landscape metrics at watershed level (black line) and local level using buffers (gray lines) with different width around the water course (blue line). The area illustrated in C corresponds to one sampled point.
We randomly chose 17 sampling sites (Fig. 2B, Table S1). Phytoplankton was collected in the water subsurface (0.3 m), in puddle formation with lower flow and higher light intensity. Periphyton was sampled from five rocks’ scraping in each stream (Schneck and Melo, 2012). The other taxonomic groups were sampled and identified using standard protocols (Vollenweider, 1974; Bellinger and Sigee, 2010). For zooplankton sampling, we filtered 300 L of water at each sampling point using a plankton net with a mesh opening of 68 µm. Fish sampling was carried out in stretches of 80 m length in each stream using single-pass electric fishing, and an alternating current generator (1000 W, 300–500 V, 1–3 A). Fishes were netted before they become immobilized, and were placed in an aerated holding container for processing. After identification and measurements, they were released into the water.
Because several taxa had very low abundance and frequency, we merged the species into groups Cladocera, Copepoda, Amoeba (Testaceae), phytoplankton, periphyton, and fish (Table S1). We calculated abundance, richness, and Shannon-Wiener diversity index (H’), and used them as response variables (Table S1).
Instream featuresWe measured several physicochemical, hydrological, and biological indicators of water quality: potential of Hydrogen (pH), Oxidation Reduction Potential (ORP, mV), electrical conductivity, Oxygen Biological Demand (OBD, mg/L), Dissolved Oxygen (DO), Nitrate (NO3, mg/L), Ammoniacal Nitrate (NH4NO3, mg/L), and turbidity (NTU). As biological indicators of water quality, we measured the number of total coliforms (NPM/100 ml) and Escherichia coli (NPM/100 ml). Water sampling, preservation, and analysis followed the standard protocols (APHA, 2005). We measured water velocity (water flux) as an indicator of streams’ hydrological characteristics.
Land cover mapTo characterize land cover at local level, we used a fine-scale land cover map performed by (Santos et al., 2021) for the study area, with 5 meters of spatial resolution corresponding to the same year of the field surveys (2017). Based on the existing drainage described in the land cover map and the drainage files from the Brazilian Institute of Geography and Statistics (IBGE in Portuguese), we specifically performed a drainage network edition for the stream features of our sampling sites. We performed the drainage edition using high-resolution Google Earth images and vectorial edition tools from Geographic Information System Quantum GIS 2.8 (QGIS Development Team, 2017).
For watershed level, we used a land cover map from MapBiomas database - collection 6 of the year 2017 (https://mapbiomas.org/) with 30 meters spatial resolution. We overlapped both maps (local land cover and MapBiomas) generating a final map with 5 meters of spatial resolution and 13 land cover categories (Fig. 2b).
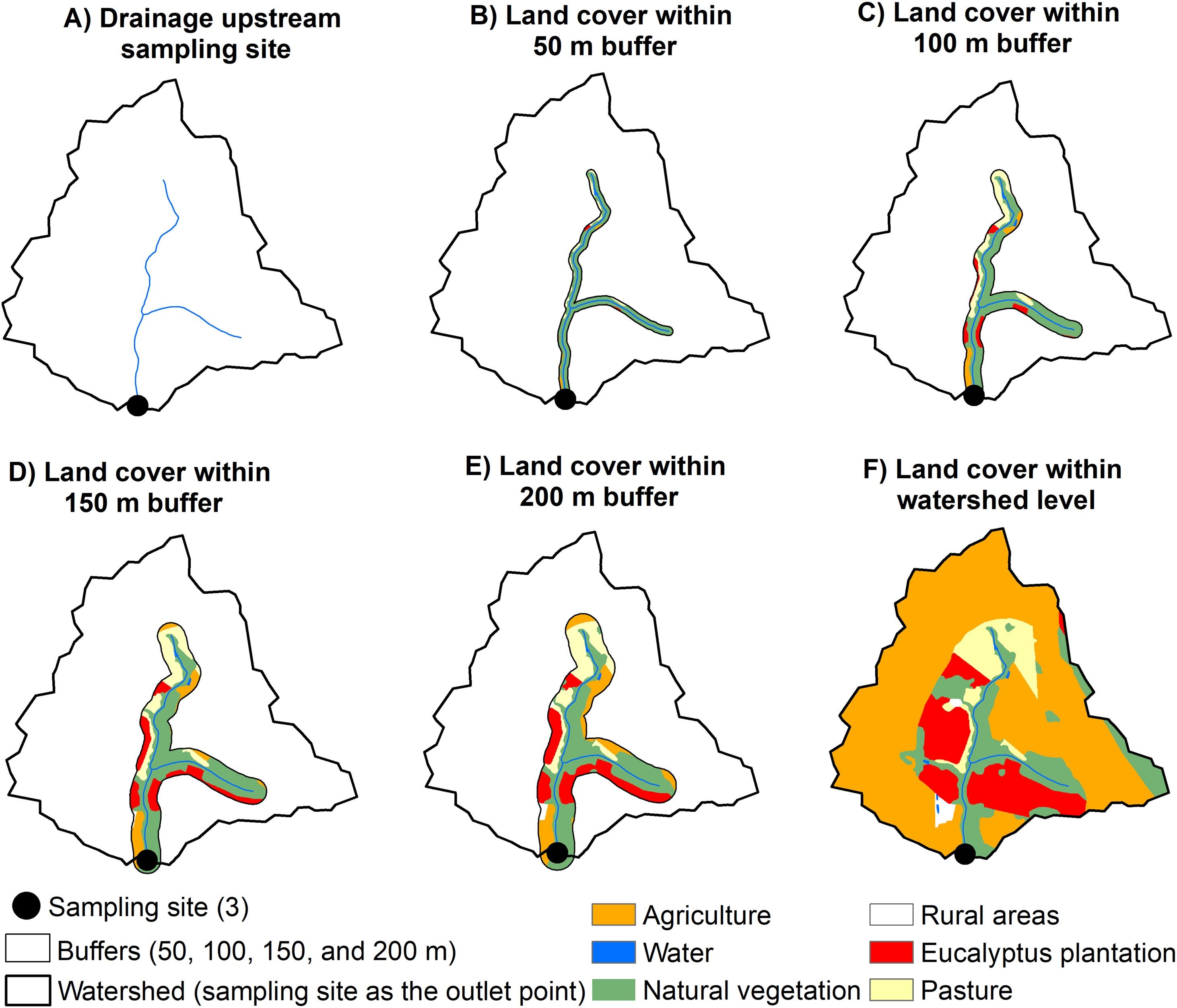
Landscape metricsWe calculated the percentage of natural vegetation cover (NV%), and landscape compositional heterogeneity (SHDI) using Shannon-Wiener Diversity Index (Table S1), at different spatial scales (Fig. 3), using the landscapemetrics package (Hesselbarth et al., 2019) implemented in R v 3.6.1. (R Core Team, 2019). For local level, we performed buffers of different width sizes (50, 100, 150, and 200 m) around the drainage upstream each sampling site (Fig. 3A–E) using QGIS.
Multi-scale approach used to estimate landscape metrics at local and watershed scales. The local scale corresponds to buffers of 50, 100, 150, and 200 m radius size designed around the upstream drainage of each sampling site. The watershed level corresponds to the area delimited upstream the drainage of each sampling site as an outlet point. (A) A sampling site and its upstream drainage. (B–E) land cover within buffers of different radius sizes performed around the upstream drainage from one sampling site. (F) land cover within the watershed of a sampling site, which was used as the outlet point to delimit the watershed.
For watershed level, we used a direction map considering the location of each sampling site as the outlet point to generate each watershed (Fig. 3A and 3F), using the function “r.water.outlet” freely available at the software GRASS (Neteler et al., 2012). The drainage direction map was derived from a digital elevation model with 30 meters of spatial resolution obtained from the Topodata website (http://www.dsr.inpe.br/topodata/).
Statistical analysesWe assessed the effect of (i) terrestrial landscape structure on freshwater community diversity and (ii) on instream features, and (iii) the effect of instream features on freshwater community diversity. For i, firstly we verified the scale-of-effect (Jackson and Fahrig, 2015) for each response variable, considering the landscape metrics NV% and SHDI calculated at each spatial scale. We defined the scale-of-effect running the Multifit function (Huais, 2018) available in the R program. We ran linear or generalized linear models (GLMs) and Akaike Information Criterion (AIC) to identify the best scale-of-effect, which corresponded to the models with the lowest AIC value (Table S2).
After that, we verified the spatial autocorrelation (SA) in response variables using the Moran’s I test implemented in ape (Paradis et al., 2004) R package. For response variables that we found spatial autocorrelation (p-value <0.05; Table S3), we included the spatial autocorrelation (SA) in the models considering the distance-weighted function of neighboring response values to model explanatory variables (Dormann et al., 2007). We used the neighborhood radius = 300 and included the SA as a new explanatory variable in the models.
For ii and iii, firstly we assessed pairwise Pearson’s correlations with ggpairs function in the ggplot2 package (Wickham, 2016) available in R program version 3.6.1. (R Core Team, 2019), to verify the multicollinearity between instream features variables, and terrestrial landscape structure. We eliminated from subsequent analyses all variables that had a coefficient of correlation (R) higher than 0.70. For instream features variables, we removed the variable nitrate that had a high correlation with turbidity and ammoniacal nitrate variables (Fig S1). For terrestrial landscape structure variables, we assessed the correlation between %NV (Fig. S2) and SHDI (Fig. S3) at different spatial scales individually, and lastly between these remaining less correlated predictors variables (Fig. S4). We kept %NV and SHDI at 50 m, 200 m, and watershed level that were less correlated. We also verified the SA between response variables in ii using the analysis described before (Table S4).
To solve i, ii, and iii, we ran Structural Equation Modelling (SEM) using the Piecewise SEM, which is a type of SEM recommended to analyze small sample size (Shipley, 2000; Lefcheck, 2016). We ran the SEM, using the PiecewiseSEM package in R program (Lefcheck, 2016). For that, we considered the terrestrial landscape structure predictors variables selected from the scale-of-effect analyses for i and the less correlated predictors variables for ii and iii.
We ran SEM for each freshwater biological group separately. For example, for Cladocera, firstly we ran SEM considering Cladocera’s richness, abundance, and Shannon-wiener diversity as response variables and terrestrial landscape structure variables as predictors. In this step, we considered all predictor variables with p < 0.05 to compose the final model.
Second, we ran SEM using instream features as response variables and terrestrial landscape structure as predictor variables. Here, we separated models by group of instreams physicochemical and hydrological, and biological indicators as response variables. We also considered all predictor variables with p < 0.05 to compose the final model. Lastly, we ran SEM using instream features as predictors and Cladocera’s richness, abundance, and Shannon-wiener diversity as response variables. We also considered all predictor variables with p < 0.05 to comprise the final model (Table S5). Finally, we ran a final model with all variables selected as good predictors in the previous models. As the final result, we considered as plausible models those with p > 0.05 in Fisher C test, and as good predictors those variables with p < 0.05 (Table S5). We performed the same steps described to Cladocera to the other freshwater biological groups. We ran linear models with Gaussian distribution for Shannon-Wiener diversity index and instream features variables, and GLMs with Poisson or negative binomial distributions for abundance and richness when data showed overdispersion (Zuur et al., 2009). We carried out all statistical analyses in the R program.
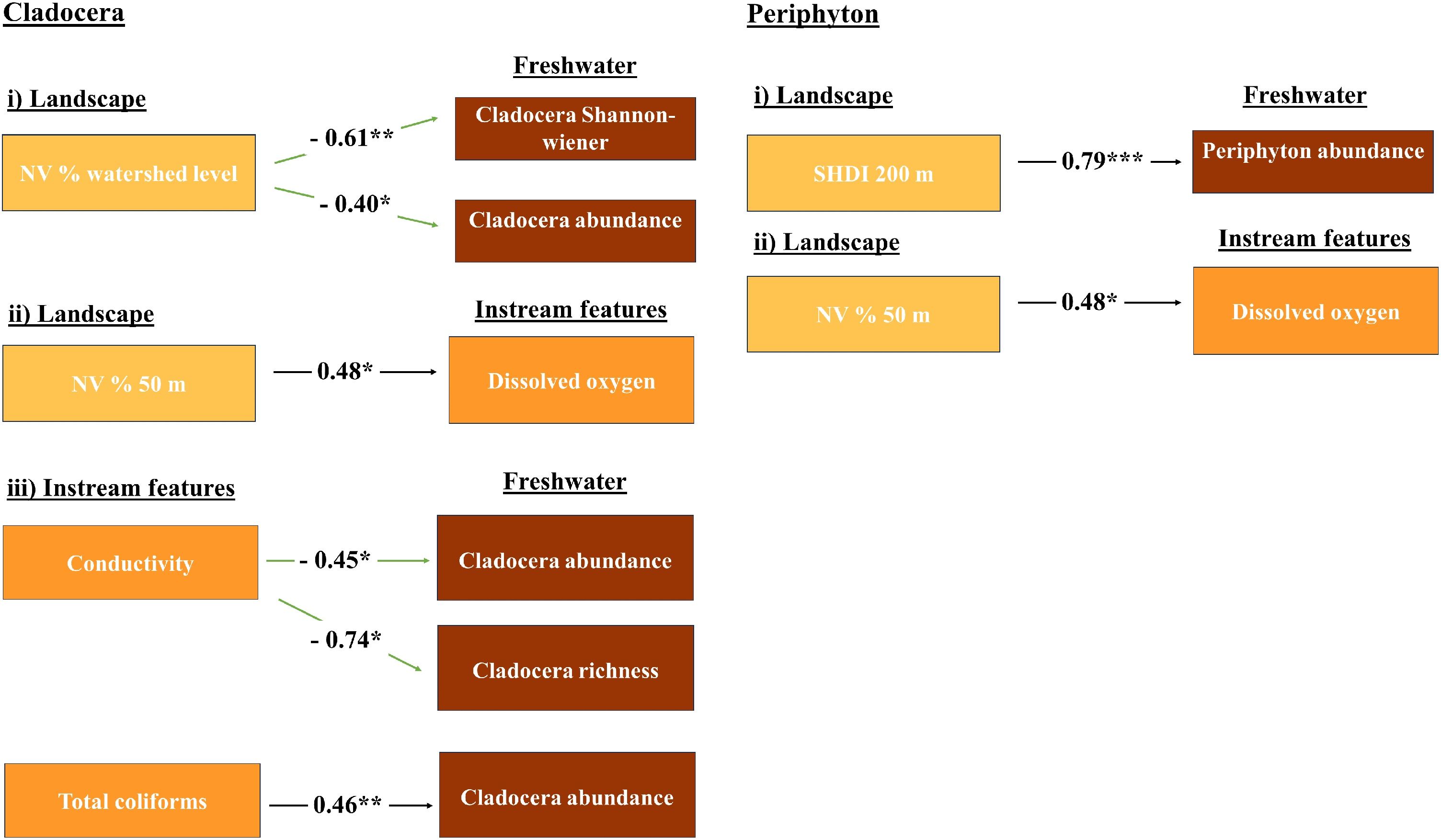
ResultsWe recorded a total of 68 Phytoplankton species (Fig. S5A), 42 Periphyton (Fig. S5B), 82 Zooplankton (Fig. S5C). For fish, we found a total of 33 species (Fig. S5D). We found no pattern between the values of scales of effect for the different freshwater communities since the scale of effect ranged from 50 m to watershed level between the different response and terrestrial landscape structure variables analyzed (Table S2). Based on SEM significant relationships, our results showed that %NV at the watershed level explained Cladocera’s Shannon–Wiener diversity (p = 0.0090) and abundance (p = 0.0216) with a negative relationship. SHDI at 200 m explained Periphyton abundance (p = 0.0000) with a positive relationship. %NV at 50 m explained dissolved oxygen (p = 0.0245) with a positive relationship. Conductivity explained Cladocera’s abundance (p = 0.0225) and richness (p = 0.0490) with a negative relationship. Total coliforms explained Cladocera’s abundance (p = 0.0015) with a positive relationship (Fig. 4). Fisher’s C was 0.19 for Cladocera’s model, and 0.75 for Periphyton’s, indicating a good model fit (p > 0.05). We found high values of R2 for most models, such as Cladocera’s richness (R2 = 0.93) and abundance (R2 = 0.94), Shannon–wiener diversity (R2 = 0.38), Periphyton’s abundance (R2 = 0.90), and dissolved oxygen (R2 = 0.24). Conversely to our expectations, we found no significant relationships between other freshwater biological groups and variables related to instream features and terrestrial landscape structure (Table S5).
Significant relationships according to structural equation modeling. (i) effects of terrestrial landscape structure on freshwater community diversity and (ii) instream features, and (iii) effect of instream features on freshwater community diversity. The signal of standardized estimate coefficients is represented by black and green arrows for positive and negative signals, respectively. %NV = percentage of natural vegetation at different spatial scales; SHDI = compositional heterogeneity at different spatial scales; * = significant effects with p < 0.05.
We found that terrestrial landscape structure (percentage of natural vegetation at the watershed level) negatively influenced Cladocera community. Microcrustaceans such as Cladocera are aquatic environmental indicators of trophic state and habitat heterogeneity due to their susceptibility to biotic and abiotic changes (Brito et al., 2020; Fernández et al., 2022). However, these microcrustaceans tend to adapt to environmental changes keeping their population growth (Dini Umi et al., 2020).
Freshwater microcrustaceans and macroinvertebrates seem to be favored in agricultural landscapes. For instance, in agricultural landscapes of the Brazilian Amazon, macroinvertebrates’ richness also decreased with the increase in vegetation amount at watershed level (Brito et al., 2020). Similarly, microcrustaceans’ richness and abundance were higher in lakes in agricultural and urban landscapes in Mexico (Fernández et al., 2022). The conversion of natural areas in other land covers can modify streams’ conditions (Bogardi et al., 2020), due to changes in light and nutrient availability, pH, temperature, and subsurface irradiance (Junk et al., 2022; Machado et al., 2016). These changes in the streams’ biotic and abiotic characteristics tend to modify the food web and primary production favoring phytoplankton and periphyton growth, which are microcrustaceans’ food sources (Dini Umi et al., 2020).
The LAND-LTER is an intensive farming landscape predominantly occupied by crops and pastures, which intensive management with high inputs of agrochemicals and fertilizers may change biotic and abiotic streams’ characteristics. Hence, we believe that this prime characteristic of our study area may explain the higher diversity and abundance of Cladocera in watersheds with low natural vegetation amount and predominantly occupied by monocultures.
Primary producer assemblages are also considered indicators of stream conditions because they are influenced by variations in environmental parameters, including land cover changes (Ferrareze, 2012). Parameters such as suspended matter, nutrient availability, and light can affect these communities (Dini Umi et al., 2020; Ren et al., 2021). The level of shading can also limit streams’ diversity since it directly affects the availability of light for primary productivity (Bott, 1983; Ghermandi et al., 2009). Here, only landscape compositional heterogeneity explained the abundance of periphyton at 200 m spatial scale. Thus, a high abundance of these organisms in heterogeneous landscapes can be due to the greater availability of light and nutrients, enabling higher growth of periphyton than in streams shaded by vegetation.
Landscapes with high levels of complexity (Estrada-Carmona et al., 2022) and compositional heterogeneity can provide diverse resources for different taxa (Fahrig et al., 2011). For instance, increasing crop heterogeneity is an effective way to increase terrestrial biodiversity in agricultural landscapes (Sirami et al., 2019). Our findings suggest that landscapes comprising different land cover types, including natural vegetation and matrices, increase the abundance of periphyton. However, it is important to highlight that, in general, streams within watersheds dominated by monocultures tend to have more suspended and dissolved material due to high levels of discharge and soil sedimentation (Bogardi et al., 2020; Mateo-Sagasta et al., 2017; Junk et al., 2022; Morabowen et al., 2019; Taniwaki et al., 2017). These characteristics can affect light availability and consequently primary producer assemblages’ growth (Dini Umi et al., 2020). In addition, it is important to note that high abundance is not always indicative of high diversity, since few species can occur in high abundance in disturbed sites leading to biotic homogenization (Siqueira et al., 2015).
We found that the percentage of natural vegetation at 50 m (%NV 50 m) positively influenced dissolved oxygen. Dissolved oxygen is an important indicator of water quality and pollution level because it indicates how much the streams are affected by residual discharge and soil sedimentation (Freitas et al., 2022; Mondal and Bhat, 2020). Also, it indicates the health of freshwater ecosystems, which is associated with high levels of dissolved oxygen. Our result showed that high %NV at fine scale can maintain high levels of dissolved oxygen, and consequently, the diversity of freshwater ecosystems, supporting ecosystems’ nutrient cycling (Taniwaki et al., 2017). Thus, our findings confirm the role of riparian forests in maintaining the health of freshwater ecosystems (Bogardi et al., 2020; Broadmeadow and Nisbet, 2004; Taniwaki et al., 2017; Valera et al., 2019). Moreover, our findings suggest that the increase in intensive farming, expansion of urban areas, and reduction of riparian forests can negatively influence instream quality and indirectly affect freshwater biodiversity.
Water conductivity is a proxy of water quality because higher water conductivity values may cause stress to aquatic biota (Taniwaki et al., 2017), due to the increase in ions carried into the streams (Zhang et al., 2019) caused by pollution and sedimentation. Our results show that Cladocera’s richness and abundance decreased in streams with higher water conductivity. We suggest that this response is related to the increase of industrial sewage, discharge of some specific nutrients from agricultural activities, as well as soil sedimentation that can affect phytoplankton growth in streams, which is the main source of food for microcrustaceans. Conversely, Cladocera’s abundance increased with the number of total coliforms. The livestock excreta and domestic waste can increase the total coliforms in streams. Thus, we believe that this group may be benefiting from livestock excreta and the lack of suitable treatment of human waste in the study area.
We analyzed a set of predictor variables from different sources and at multiple spatial scales. However, we found no significant relationships between landscape and instream features and freshwater biological groups such as copepods, amoebas, phytoplankton, and Pisces. Stream conditions change over time, making time an important predictor of freshwater biodiversity integrity (Green et al., 2022). Our data were collected during a single period, considering that natural and anthropogenic factors can alter water flow intensity (Bogardi et al., 2020), which is a key factor influencing biological responses in streams (Goździejewska et al., 2024). This lack of data from different time periods can explain the lack of significant effects for some groups. Furthermore, other variables such as habitat complexity and quality, elevation (Lemke and Súarez, 2013; Green et al., 2022), latitude, longitude (Green et al., 2022), and food resources (Arrieira et al., 2015; Goździejewska et al., 2024) can be more important in explaining these groups. Therefore, we suggest that future studies investigate additional relationships across different periods.
Implications for conservationOur findings suggest that freshwater biological groups are benefiting from economic activities occurring in the LAND-LTER landscape. However, it’s important to highlight that this result is particularly associated with two biological groups such as Cladocera and Periphyton, which can indicate that an increase in economic activities can cause freshwater biotic homogenization, prioritizing biological groups that tend to specifically adapt to habitat loss, inappropriate discharge of human waste, and livestock excreta. In contrast, Cladocera’s richness and abundance decreased with higher conductivity values, demonstrating that sedimentation of streams tends to affect this group.
We also found that the percentage of native vegetation at a fine scale can increase the amount of dissolved oxygen in streams. The dissolved oxygen is an essential element to maintain freshwater life in streams. Thus, our findings emphasize the importance of Brazilian environmental law compliance (Dala-Corte et al., 2020; Valera et al., 2019), which ensures the conservation of riparian forests at fine scale and other areas of natural vegetation such as Legal Reserves inside private lands to increase the amount of natural vegetation areas at the watershed level.
In the LAND-LTER landscape, land use and land cover spatial patterns are different in the south and north, due to slope differences (Santos et al., 2021). The south has a flatter relief and is dominated by highly intensive agriculture with few and small natural vegetation areas, while the north has a rugged relief, and is characterized by a mosaic of land uses such as livestock and agriculture, with the highest number of natural vegetation areas, including riparian forests. In this study, we reinforce the need for environmental guidelines to protect and restore natural vegetation areas to diversify the mosaic, mainly in the south. Increasing technical assistance and rural credit in the north is essential to guide and incentivize farmers to adopt good and sustainable management practices to reduce the contamination and pollution of streams and soil sedimentation.
For the same landscape, we found that riparian forests are essential features to maintain the terrestrial biodiversity in the Cerrado agricultural landscapes (Santos et al., 2022; Martello et al., 2023; Silveira et al., 2023). Here, we also underscore the importance of these ecosystems to keep water quality within this landscape. Furthermore, we are getting attention for a problem related to freshwater biodiversity loss and water quality sinking in this landscape.
FundingThis work was supported by grants given to the LAND-LTER research network, supported by FAPEG (project nº 201710267000331 and 202010267000404), CNPq (project nº and 445419/2024-5, and PPBio Biota Cerrado funded by CNPq (project nº 441166/2023-7).
Data availabilityAdditional data are provided as supporting information in the online version of this manuscript.
The authors declare that they have no conflict of interest.
JSS received a postdoctoral grant from the São Paulo Research Foundation (FAPESP, process nº 2019/09713-6 and 2022/00166-5), CAPES-Print scholarship (process nº 88887.890889/2023-00), and a postdoctoral grant from National Council for Scientific and Technological Development (CNPq, process nº 179354/2024-8). JCN thanks FAPEG (process nº 201710267000519) and UEG (process nº 202300020021777) for their continued support of aquatic ecology research. FM received a postdoctoral fellowship from São Paulo State University, UNESP Rio Claro/AGRUP. RGC was supported by a Visiting Research fellowship from São Paulo Research Foundation (FAPESP, process nº 2022/10760-1). KBM received a FAPEG postdoctoral fellowship (FAPEG, process nº 202110267000885). RGC, FBT (process nº 312844/2021-2), LCGV, and JCN (process nº 303181/2022-2) have been continuously supported by productivity grants from CNPq, which we gratefully acknowledge.