Climate change and biological invasion are major threats to biodiversity, but their combined effects have rarely been quantified. The introduction of congeneric non-native species, in particular, can be especially problematic for native species due to competition and hybridization. Here, we quantify the impacts of climate change on the distribution of an ecologically and economically important native species, Euterpe edulis, and on the invasion potential of its congeneric E. oleracea, across the Atlantic Forest biodiversity hotspot. We modelled current and future environmental suitability for both species, using a comprehensive set of algorithms and climatic scenarios, and quantified the extent of overlap of their environmentally suitable areas. Climate change reduced environmental suitability for E. edulis, but had neutral effects on E. oleracea. Current and future overlap areas, where competition and hybridization are more likely, were concentrated mostly in the southeastern region of Brazil. Our results suggest that native and non-native congeneric species respond differently to climate change, and that climate change and the introduction of E. oleracea are additional threats to the threatened E. edulis. We recommend avoiding new introductions of E. oleracea especially in the southeastern portion of the Atlantic Forest, and the maintenance of protected areas especially in the southern region.
Biological invasions are one of the main threats to biodiversity globally (Richardson and Pyšek, 2008; Bellard et al., 2016). Invasive species may lead to enormous ecological and economic losses, which usually become increasingly difficult and costly to repair (Pimentel et al., 2005). Indeed, most control programs have failed in extirpating non-native species after their spread throughout a new area, evidencing the importance of predicting invasion potential before species are introduced in new areas (Thuiller et al., 2005; Epanchin-Niell and Hastings, 2010). To do so, it is increasingly necessary to consider climate change, as this process may change invasion potential of non-native species (Bradley et al., 2010; Hulme, 2017). Some studies have shown that climate change may increase the fecundity, growth and/or survival of non-native individuals, facilitating their spread across new areas (Walther et al., 2009; Bellard et al., 2013). Importantly, climate change may also affect native species directly, in some cases reducing their fitness, abundance and competitiveness against invaders, thus increasing the vulnerability of ecosystems to invasion (Sorte et al., 2013; Menezes-Silva et al., 2019). Therefore, identifying areas with higher invasion risk, and predicting how both native and non-native species will respond to climate change, are essential tasks to guide management actions (Thuiller et al., 2005; McGeoch et al., 2016).
The invasion potential of non-native species depends on many factors, including their ecological, physiological and evolutionary traits (Rejmánek, 2011; Mathakutha et al., 2019). The preadaptation hypothesis predicts that non-native species that are closely related to native species have a higher probability of becoming invasive in a local community (Darwin, 1859; Thuiller et al., 2010), as they may have similar pre-adaptations, environmental requirements, and mutualistic interactions with the local biota (Wiens and Graham, 2005; Cadotte et al., 2018). In contrast, Darwin's naturalization hypothesis predicts that non-native species have lower invasion potential when they are closely related to native species, since they may share natural enemies and strongly compete for resources due to a higher niche overlap (Darwin, 1859; Park and Potter, 2013). These two long-standing hypotheses, referred to as “Darwin's naturalization conundrum” (Diez et al., 2008), can operate simultaneously in nature, with non-native species becoming successful invaders due to shared traits, but also promoting the extinction of closely-related native species due to a severe competition (Diez et al., 2008; Cadotte et al., 2018).
The introduction of closely-related non-native species, such as congenerics, can be especially problematic in taxa with weak reproductive barriers, since they can hybridize with native species, producing fertile descendants that could backcross with parental species (Prentis et al., 2007). This hybridization process can result in the erosion of native genomes along generations, reducing genetic diversity (Meilink et al., 2015). Also, the introduction of exotic alleles may decrease the fitness of native species, since adapted genes could be extirpated (Rhymer and Simberloff, 1996; Prentis et al., 2007). On the other hand, the invasiveness of an introduced species could be increased due to the introgression of locally adapted genes from native species (Ellstrand and Schierenbeck, 2000; Hovick and Whitney, 2014).
The negative effects of congeneric non-native species may occur especially in areas that are environmentally suitable to both species, as environmental suitability is usually correlated to population abundance (Weber et al., 2017). Therefore, identifying potential overlap areas, i.e. areas that are environmentally suitable to both native and non-native species, is important to infer which areas are under higher potential risk associated with the introduction and spread of non-native species (Ben Rais Lasram and Mouillot, 2009). In addition, understanding whether climate change will alter the extent and spatial distribution of overlap areas is crucial, to predict if potential invasion impacts are likely to increase in the future.
Here, we quantify the impacts of climate change on a threatened native species and on the invasion potential of a congeneric non-native species, in the Atlantic Forest, a top-ranked biodiversity hotspot (Rezende et al., 2018). We focus our analysis on the threatened native palm Euterpe edulis, an ecologically and economically important palm listed as Vulnerable (Martinelli and Moraes, 2013). In addition to deforestation and palm heart harvest (Souza and Prevedello, 2019, 2020), E. edulis currently faces two additional potential threats, represented by climate change and the introduction of the congeneric palm Euterpe oleracea in the Atlantic Forest (Bovi et al., 1987; Campos et al., 1991; Tiberio et al., 2016). Euterpe oleracea is native from the Amazon, and can compete and hybridize with E. edulis, leading to further reductions in the abundance of this important native palm (Tiberio et al., 2016). Therefore, quantifying current and future environmental suitability for these two species across the Atlantic Forest is central to understand how climate change and the introduction of E. oleracea may impact the already threatened E. edulis.
Specifically, we aim to: (i) quantify current environmental suitability for both species across the Atlantic Forest; (ii) determine if climate change will affect environmental suitability for the native E. edulis, and invasion risk of the non-native E. oleracea; (iii) assess the spatial variation of environmental suitability across the Atlantic Forest, and which climatic variables drive such changes; (iv) determine if climate change will alter the extent of spatial overlap between the environmentally suitable areas of both species.
Materials and methodsStudy speciesEuterpe edulis occurs mainly in Brazil, primarily in the Atlantic Forest but also in gallery forests of the Cerrado, and also in Argentina and Paraguay (Henderson et al., 1995). The fruits of this species are considered as a keystone resource consumed by a great variety of animals (Galetti et al., 1999). In addition, E. edulis palm heart is an economically important non-timber forest product, whose extraction has led to strong reductions in abundance (Souza and Prevedello, 2020), as it kills individuals of this single-stemmed palm (Silva-Matos et al., 1999; Martinelli and Moraes, 2013). Recently, pulp production from E. edulis fruits has been encouraged as a more sustainable economic activity for local farmers (Ball and Brancalion, 2016).
Euterpe oleracea Mart. occurs in Brazil, Colombia, Ecuador, French Guiana, Guyana, Suriname, Trinidad-Tobago and Venezuela. In Brazil, this species is native from the Amazon, but currently it also occurs in the Atlantic Forest due to human introductions (Henderson et al., 1995). This multi-stemmed palm has a valuable economic value as a sustainable source of palm heart, due to its ability of resprouting after the extraction of the apical meristem (Pollak et al., 1995). In addition, the fruit pulp of this species is also considered an important non-timber forest product (“açaí’; Henderson et al., 1995). The first reported introduction of E. oleracea in the Atlantic Forest occurred around 1950, for ornamental purposes (Bovi et al., 1987). In the late 1970s, E. oleracea was further introduced in the Atlantic Forest as a sustainable alternative to palm heart harvest of E. edulis, in an attempt to protect the native species due to its inability of resprouting (Henderson et al., 1995). In the following years, the cultivation of E. oleracea was also improved in the Atlantic Forest, due to fruit harvest for “açaí” production, and the cultivation of hybrids with E. edulis for heart palm harvest (Campos et al., 1991; Tiberio et al., 2016). In the Atlantic Forest, both species show an overlap in their fructification period, and share at least nine bird dispersers (Tiberio et al., 2016). In addition, these species are able to generate hybrids in the field that can reach the reproductive stage with the production of viable seeds (Bovi et al., 1987; Campos et al., 1991).
Occurrence recordsWe obtained occurrence records for both species mainly from Global Biodiversity Environmental Facility, accessed from R 4.0.2 (R Core Team, 2020) via the rgbif package (Chamberlain, 2017) on 2020-16-04. We complemented these records with data from Henderson and Galeano (1996) and three online databases (http://jabot.jbrj.gov.br; http://reflora.jbrj.gov.br; and http://sweetgum.nybg.org/science). For both species, we retained both records within and outside the focal study area (Atlantic Forest), to estimate the maximum potential environmentally suitable area for each species as recommended by Jimenez-Valverde et al. (2011). For both species, we retained only records located at least 10km away (two times the resolution of the environmental variables – 5km; see next section) to maximize their independence, resulting in a total of 111 and 150 valid occurrence records for E. edulis and E. oleracea (Fig. S1).
Environmental variablesWe obtained bioclimatic variables and elevation from WorldClim 2.1 (Fick and Hijmans, 2017) at 2.5min (∼5km) resolution. We first obtained all 19 bioclimatic variables representing current (∼1970–2000) conditions. To select bioclimatic variables for modelling, we extracted the current values for all variables at 1000 random points within the modelling area of each species. The modelling area was defined as the minimum convex polygon encompassing all occurrence records of each species plus a boundary strip of 200km (as in Souza and Prevedello, 2020). We then retained only variables with low correlation (r<0.65). These procedures were ran separately for each species, resulting in six bioclimatic variables for each species (E. edulis: maximum temperature of warmest month, temperature annual range, mean temperature of wettest quarter, annual precipitation, precipitation of wettest quarter and precipitation of coldest quarter; E. oleracea: mean diurnal range, temperature seasonality, mean temperature of wettest quarter, precipitation of wettest month, precipitation seasonality and precipitation of warmest quarter).
We then obtained the predicted values of each variable for the future (∼2070; 2061–2080), from five different global climate models (GCMs; BCC-CSM2-MR, CanESM5, IPSL-CM6A-LR, Miroc6 and MRI-ESM2-0), all assuming a shared socio-economic pathway of 8.5 (Fick and Hijmans, 2017). These models vary in their ability to correctly predict temperature and precipitation in tropical areas, but in general have a relatively good performance (Anav et al., 2013; Yin et al., 2013). By combining the predictions of these different GCMs, our models were more likely to produce general estimates of environmental suitability not restricted to a particular GCM.
Environmental suitability modellingAll steps of the environmental suitability modelling were equal for E. edulis and E. oleracea, and were ran separately for each species. We first estimated current environmental suitability within the modelling area, using five algorithms encompassing different modelling approaches and assumptions (BIOCLIM, GLM, MaxEnt, Random Forest and SVM). For BIOCLIM, we used as training data only the occurrence records of each species. For MaxEnt, we used as training data the occurrence records plus 10,000 “background” points randomly chosen within the distribution area of each species. For the other three models (GLM, Random Forest and SVM), we used as training data the occurrence records plus 10 “pseudo-absence” points for each record, totaling 1110 and 1500 pseudo-absences for E. edulis and E. oleracea, respectively. Pseudo-absence points were randomly chosen from raster cells located within the modelling area of each species, but outside the environmentally suitable area estimated from BIOCLIM (following Lobo and Tognelli, 2011).
For each algorithm, we used 10-fold cross-validation, repeated 10 times, splitting data into 90% training and 10% test (Elith and Leathwick, 2009). For model testing, we used the 10% remaining presences and also pseudo-absences (generated as explained above), for all algorithms. We evaluated the performance of each model by calculating the true skill statistic (TSS) and the area under the curve (AUC), separately for each iteration and algorithm. We only retained as “valid models” the models with TSS>0.70 and AUC>0.85. We then used each of the valid models to produce separate maps of predicted environmental suitability across the focal study area (the Atlantic Forest), separately for current (one map) and future climatic conditions (five maps, one for each GCM).
To convert each of the resulting continuous suitability maps to binary maps, we used the maximum training specificity and sensitivity threshold method, which has shown good performance in simulation tests (Liu et al., 2005, 2013) and has been commonly used to assess climate change impacts on species (e.g. Carvalho et al., 2015; Hu et al., 2020). To be able to ensemble the continuous suitability maps produced by each of the five different algorithms, we first standardized pixel values to vary between zero and one separately for each map. We then ensembled the valid current suitability maps produced by the 10 different iterations of each algorithm, by calculating the TSS-weighted mean of individual model outputs, obtaining a single final map of current continuous suitability. The same approach was applied to ensemble the valid future suitability maps, separately for the five GCMs. Finally, we calculated a simple average of the five future suitability maps, to produce a single final map of future continuous suitability. A similar approach was used to ensemble binary suitability maps, but using the majority ensemble rule instead of TSS-averaging (Araújo and New, 2007). Taken together, all these procedures resulted in eight suitability maps, one binary and one continuous for each species (E. edulis and E. oleracea) in each time period (current and future), which were used in further analyses. We focused all analyses only on the average maps, which were sufficient to answer our ecological questions; exploring variation across algorithms and GCMs (i.e. uncertainty) was beyond the scope of this study.
Data analysisTo test for climate change impacts on environmental suitability for each species, we first selected a random subset of Atlantic Forest raster cells (Fig. S2). All statistical tests were based on the 96 selected cells, rather than on all raster cells, to reduce spatial autocorrelation and the probability of Type I error. To compare current versus future continuous suitability values, we used a one-sample t-test, separately for each species. The data used in the test were the 96 environmental suitability change values, i.e. the future minus the current environmental suitability per cell. Positive change values indicated an increase in environmental suitability. To compare current versus future binary values, we applied a chi-square goodness-of-fit test to the binary vales of the 96 cells, testing for a significant association between time period (current versus future) and binary suitability (suitable versus unsuitable).
To test whether the continuous change values differed across forest physiognomies, we classified each of the 96 values into one of the three main forest physiognomies of the Atlantic Forest: dense (n=16 cells), seasonal (n=42) and mixed forests (n=7; IBGE, 2012; Fig. S2). These three physiognomies cover about 82% of the biome (Fig. S2). Seasonal forests included both deciduous and semi-deciduous forests, as deciduous forests occupied only a small part of the modelling area. We compared change values between physiognomies using a one-way analysis of variance followed by a Tukey's HSD post hoc test to assess significant pairwise differences. This test was only applied to E. edulis, as the changes in environmental suitability were not statistically significant for E. oleracea (see Results).
To evaluate the relative importance of the bioclimatic variables as drivers of the environmental suitability changes observed for E. edulis, we used a linear multiple regression. The dependent variable was suitability change, and the predictors were the changes in the six bioclimatic variables, calculated as future minus current values, for each of the 96 cells. We standardized predictors using the z-transformation, to be able to assess variable importance based on the resulting model coefficients. The larger the absolute coefficient, the higher the variable importance.
Finally, to test whether climate change will affect the overlap area between E. edulis and E. oleracea (i.e. the area environmentally suitable for both species), we applied a chi-square goodness-of-fit test, to test for significant association between time period (current versus future) and overlap occurrence (yes–the cell is suitable for both species- versus no–the pixel is unsuitable for at least one species).
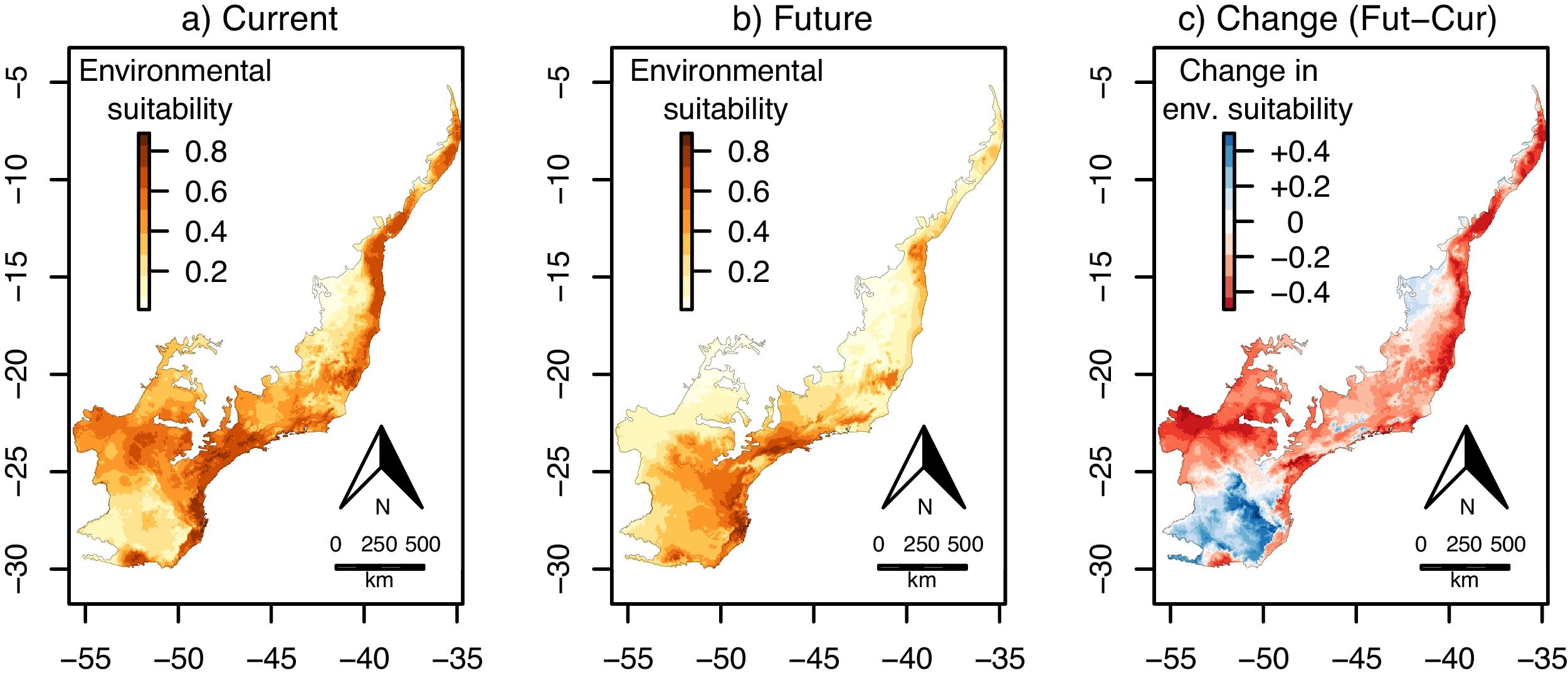
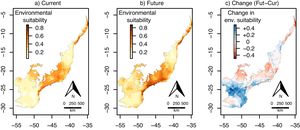
ResultsEuterpe edulisThe environmental suitability models had a very good performance for E. edulis (AUC and TSS mean±SD=0.97±0.02 and 0.91±0.06, respectively). Current environmental suitability for this species across the Atlantic Forest varied from 0.008 to 0.89 (Fig. 1a), whereas future environmental suitability varied from 0.07 to 0.72 (Fig. 1b).
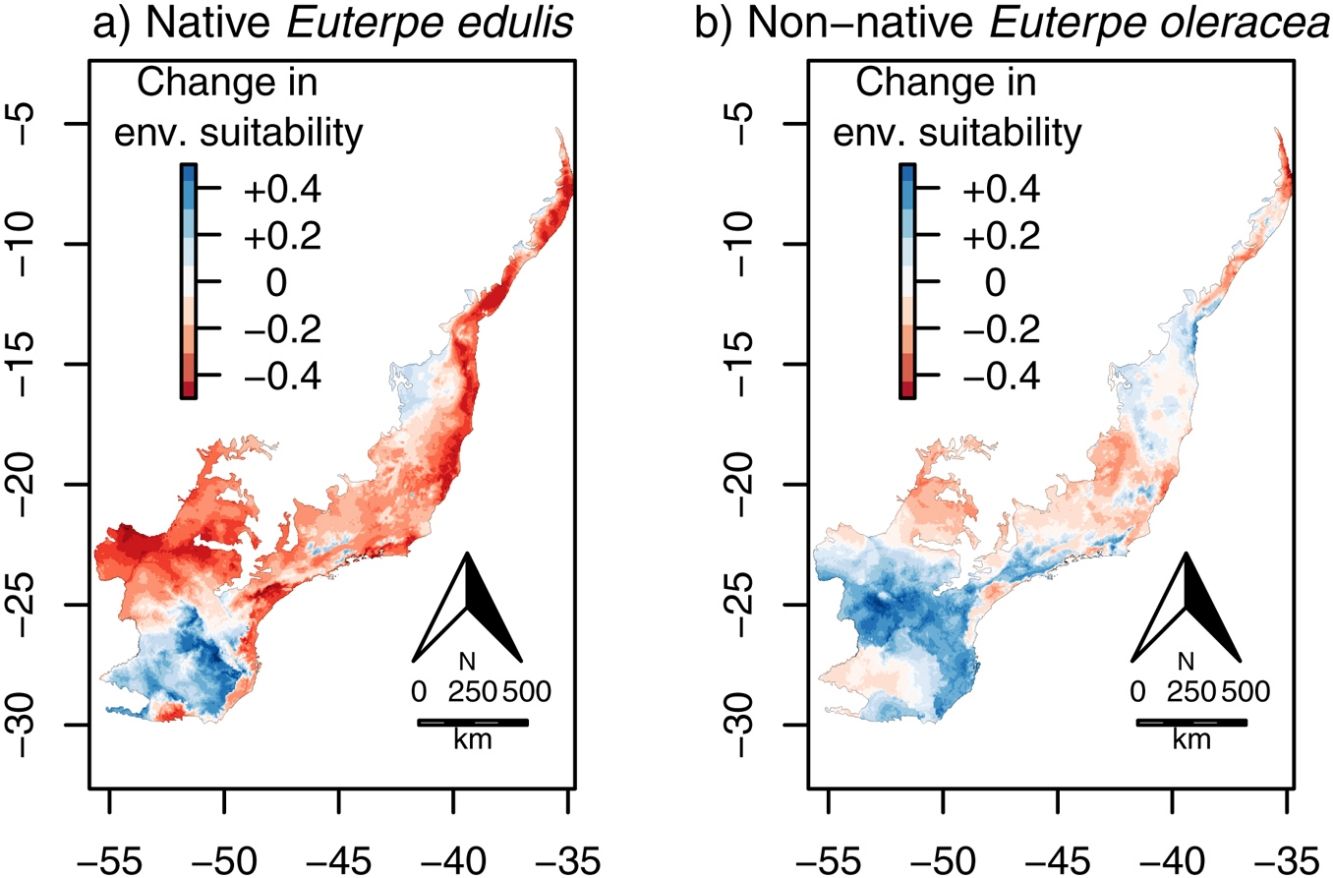
Continuous environmental suitability for Euterpe edulis across the Atlantic Forest. (a) Current suitability; (b) future (2070) suitability; (c) change in suitability (future - current). In (c), positive (blue) and negative (red) values indicate increases and decreases in climatic suitability, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
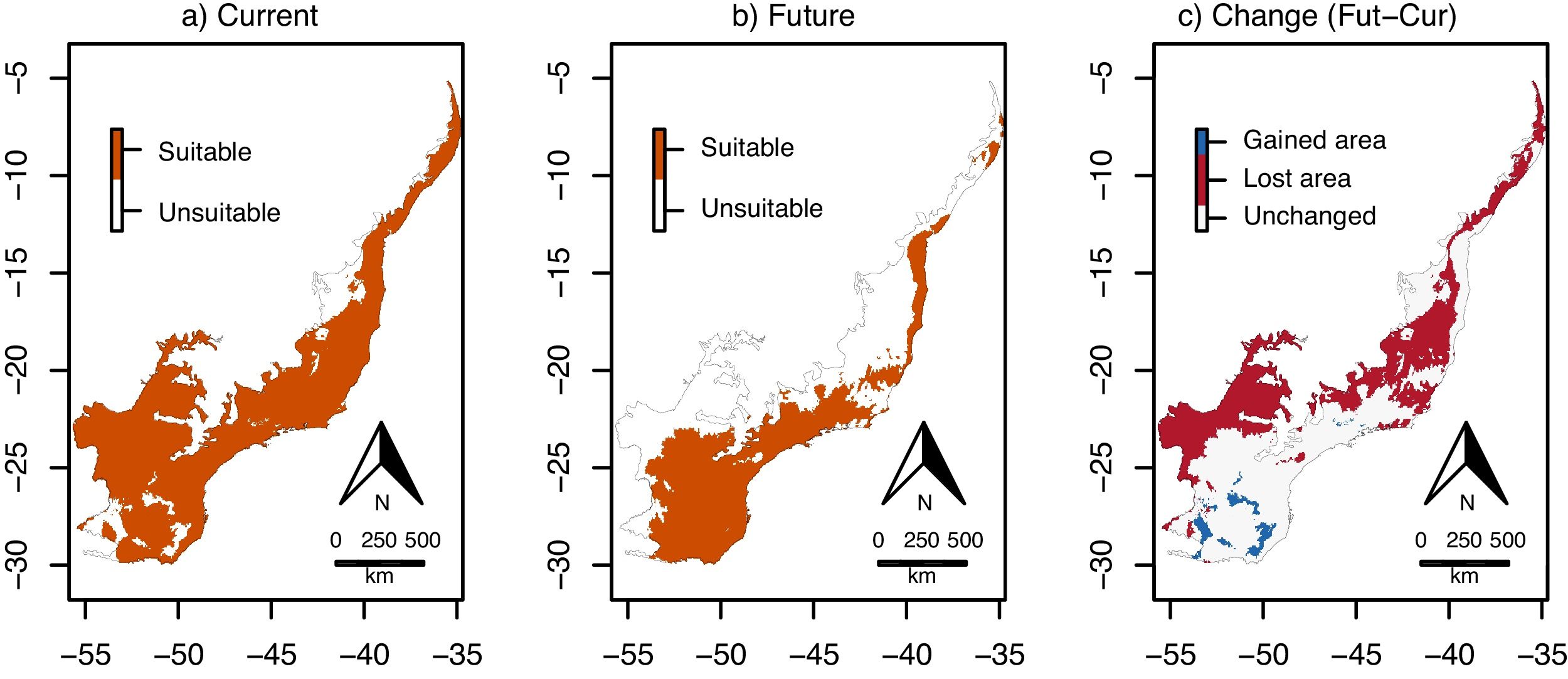
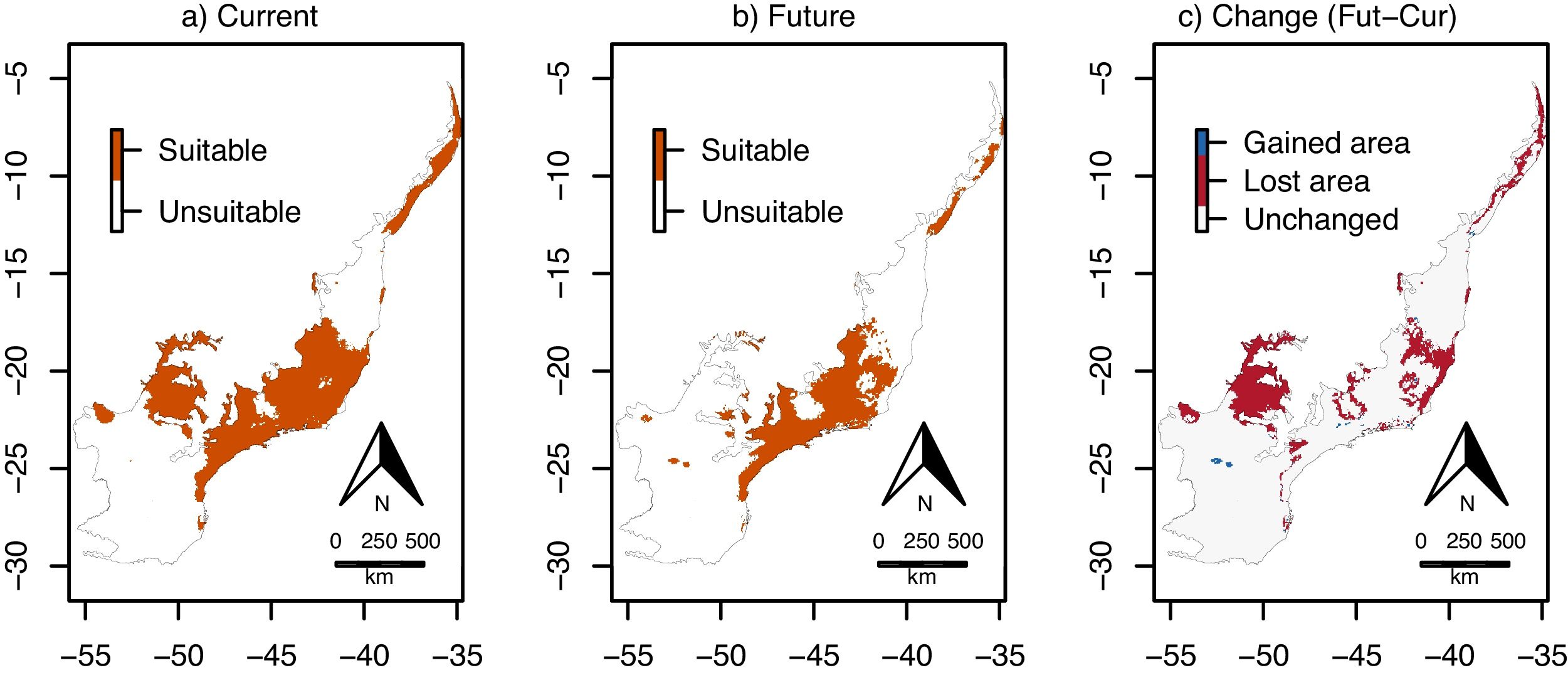
Climate change was estimated to reduce environmental suitability for E. edulis significantly, in −15.7% on average, across the Atlantic Forest (Fig. 1c; 95%CI=−19.1 to −12.3%; t=−9.1, df=95, p<0.001). Similarly, the binary projections indicated that climate change will reduce the environmentally suitable area for the species in −43.4%, from 103,675,950ha to 58,644,000ha (Fig. 2a–c). This reduction was statistically significant, as indicated by the significant association between time period (current versus future) and environmental suitability (suitable versus unsuitable; χ=33.10, df=1, p<0.001).
Environmentally suitable areas for Euterpe edulis across the Atlantic Forest. (a) Current suitable area; (b) future (2070) suitable area; (c) change in suitable area (future–current). Maps 2a and 2b are the binary version of maps 1a and 1b, respectively, with each pixel classified as either environmentally suitable (orange) or unsuitable (white) for the species. In (c), red pixels are suitable under current conditions but will be unsuitable in the future; blue pixels are unsuitable under current conditions, but will become suitable in the future; and gray pixels will maintain their current status (environmentally suitable or unsuitable). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
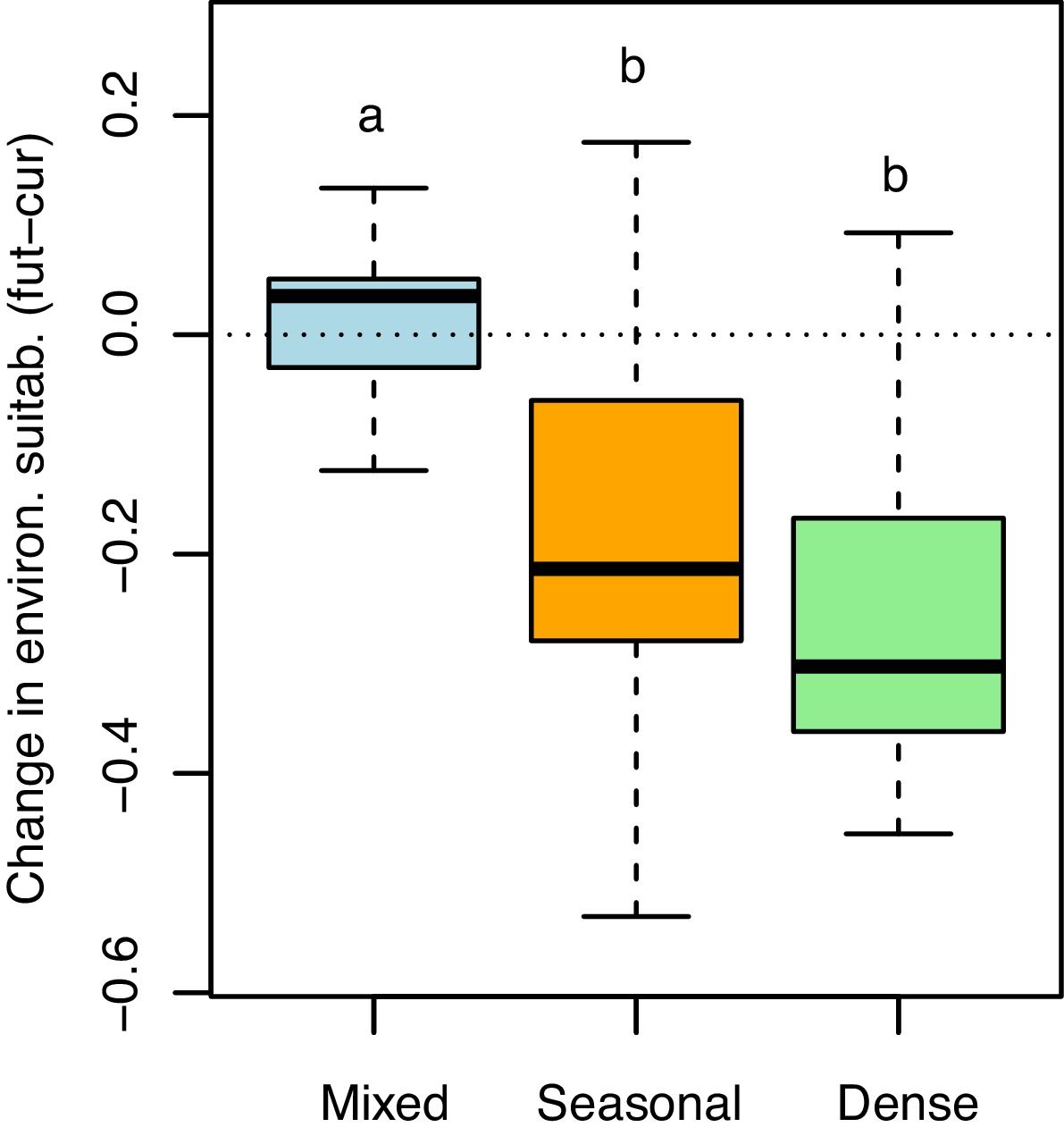
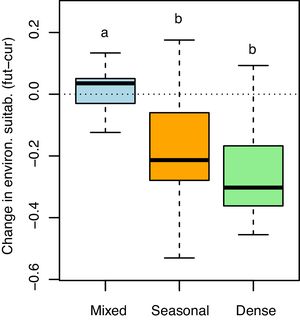
Both the direction and magnitude of environmental suitability change (i.e. future minus current suitability values; see Fig. 1c) for E. edulis differed among forest physiognomies (F2,62=7.97, P=0.001; Fig. 3). Change values were slightly positive on average in mixed forests (mean±SD=0.01±0.08), but negative and significantly lower in both seasonal (−0.17±0.15) and dense forests (−0.25±0.14; Tukey tests P: mixed vs seasonal=0.01, mixed vs dense=0.001, seasonal vs dense=0.16). The binary projections indicated that the change in suitable area will be significant only in the seasonal forest (χ=30.65, df=1, p<0.001), but not in the mixed (χ=0.01, df=1, p=0.99) and dense forests (χ=1.47, df=1, p=0.22).
Changes in environmental suitability for Euterpe edulis across three physiognomies of the Atlantic Forest (mixed, seasonal and dense forests). Changes were calculated as the difference between future (2070) minus current continuous suitability values, considering only 96 map pixels located at least 100km apart across the Atlantic Forest, to maximize independence. Positive values indicate an increase in suitability with climate change. The horizontal dotted line indicates no change (=0). Boxplots with different letters are statistically different (P<0.05, Tukey test).
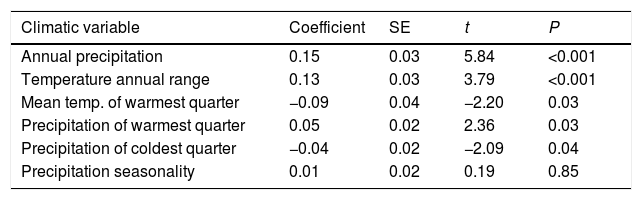
The changes in environmental suitability were mainly associated to changes in annual precipitation, temperature annual range, and mean temperature of warmest quarter, in this order (Table 1; Fig. S3). Environmental suitability increased significantly with increased annual precipitation and temperature annual range, and with decreased mean temperature of warmest quarter (Table 1; Fig. S3).
Climatic drivers of environmental suitability change for Euterpe edulis across the Atlantic Forest. Environmental suitability change was calculated from the continuous suitability maps (future–current), and used as the dependent variable. Bioclimatic variables (standardized to a mean of 0 and standard deviation of 1) were used as predictors. The coefficients indicate the direction (positive or negative) and relative importance of the effects of each variable on environmental suitability change. The analysis was run considering only 96 map pixels located at least 100km apart to maximize independence.
| Climatic variable | Coefficient | SE | t | P |
|---|---|---|---|---|
| Annual precipitation | 0.15 | 0.03 | 5.84 | <0.001 |
| Temperature annual range | 0.13 | 0.03 | 3.79 | <0.001 |
| Mean temp. of warmest quarter | −0.09 | 0.04 | −2.20 | 0.03 |
| Precipitation of warmest quarter | 0.05 | 0.02 | 2.36 | 0.03 |
| Precipitation of coldest quarter | −0.04 | 0.02 | −2.09 | 0.04 |
| Precipitation seasonality | 0.01 | 0.02 | 0.19 | 0.85 |
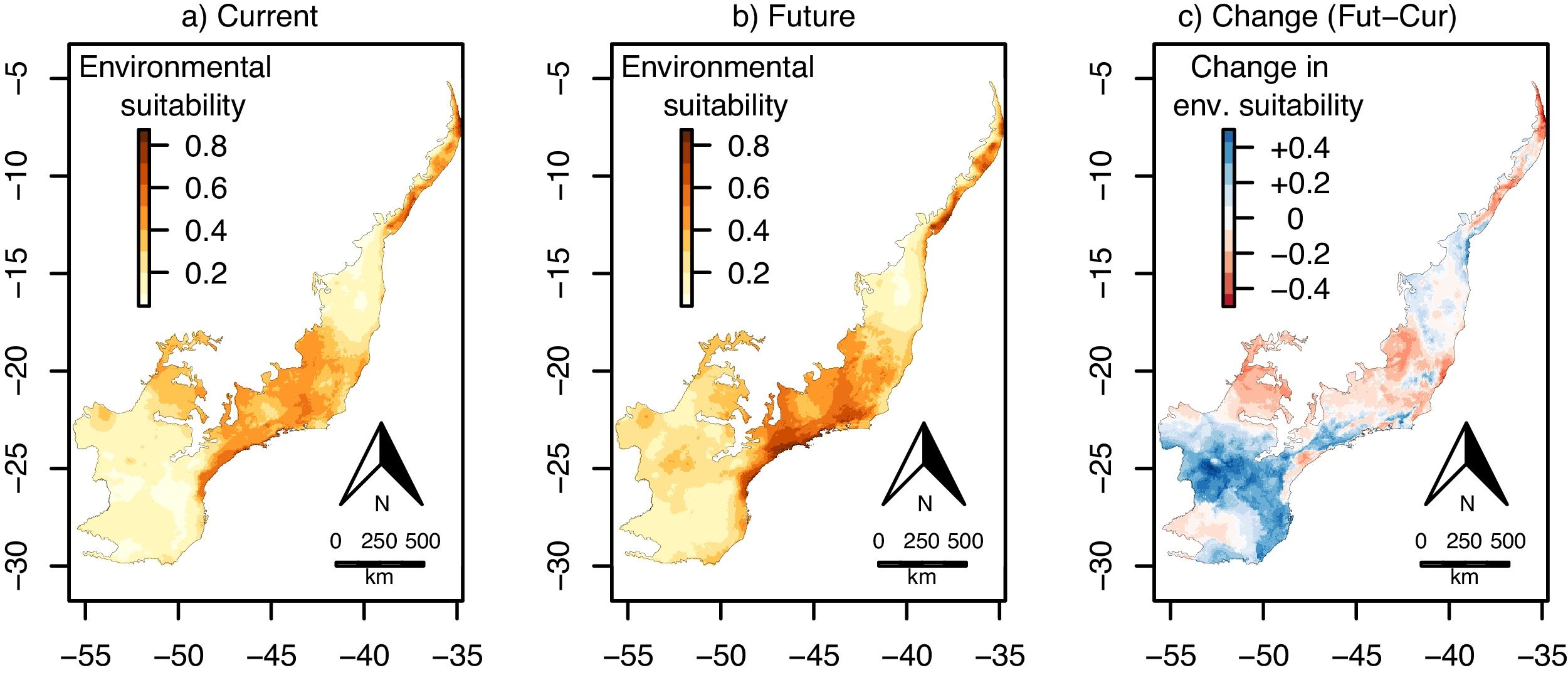
The environmental suitability models also had a very good performance for E. oleracea (AUC and TSS mean±SD=0.95±0.04 and 0.85±0.10. Current environmental suitability across the Atlantic Forest varied from 0.04 to 0.87 (Fig. 4a), whereas future environmental suitability varied from 0.06 to 0.65 (Fig. 4b).
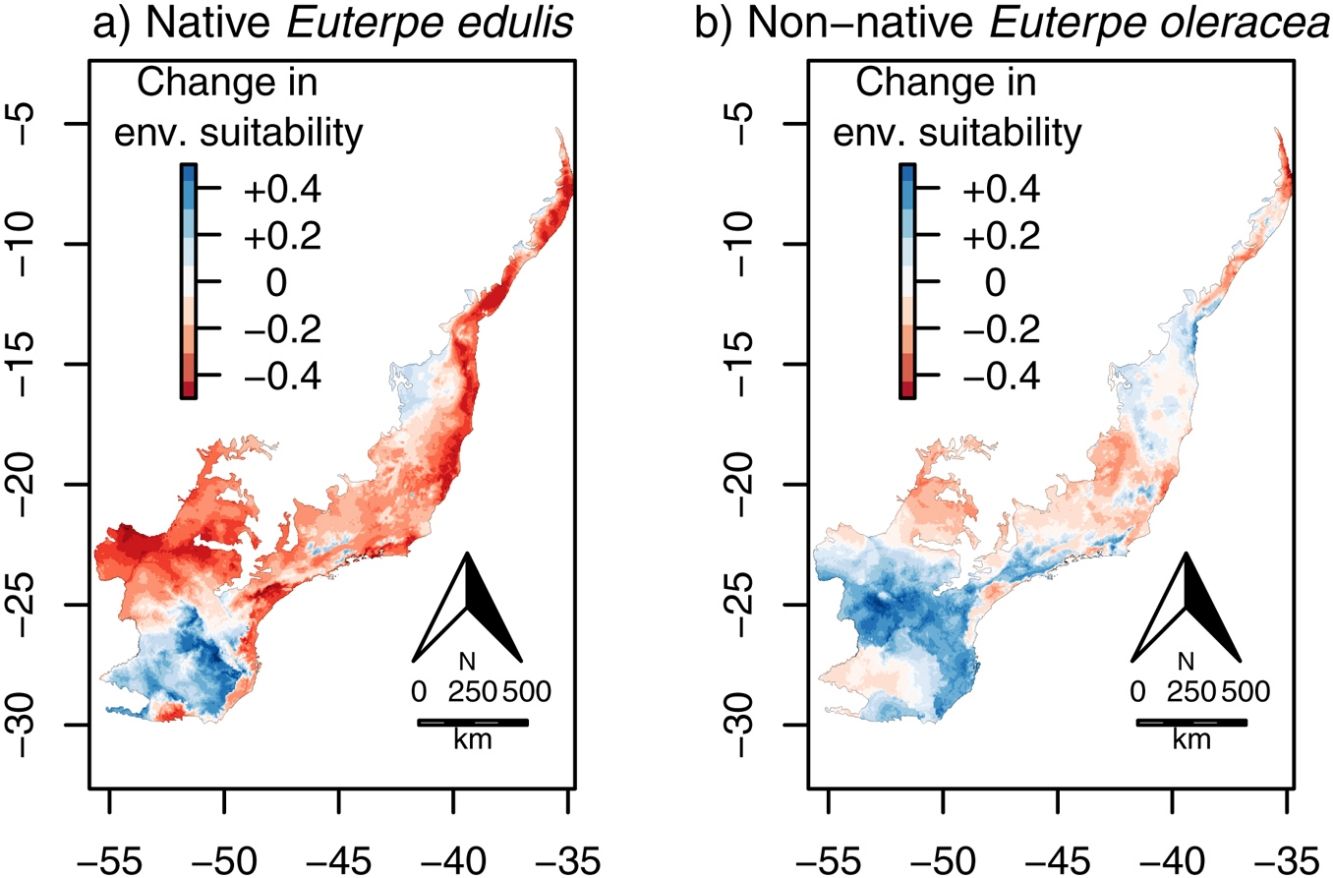
Continuous environmental suitability for Euterpe oleracea across the Atlantic Forest. (a) Current suitability; (b) future (2070) suitability; (c) change in suitability (future–current). In (c), positive (blue) and negative (red) values indicate increases and decreases in climatic suitability, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Contrary to E. edulis, for E. oleracea there were no significant effects of climate change on environmental suitability, regardless whether considering the continuous (mean change=−0.01, CI=−0.03 to 0.002; t=−1.63, df=95, p=0.11; Fig. 4c) or the binary projection (χ=3.18, df=1, p=0.08; Fig. 5a–c).
Environmentally suitable areas for Euterpe oleracea across the Atlantic Forest. (a) Current suitable area; (b) future (2070) suitable area; (c) change in suitable area (future–current). Maps 5a and 5b are the binary version of maps 4a and 4b, respectively, with each pixel classified as either environmentally suitable (orange) or unsuitable (white) for the species. In (c), red pixels are suitable under current conditions but will be unsuitable in the future; blue pixels are unsuitable under current conditions, but will become suitable in the future; and gray pixels will maintain their current status (environmentally suitable or unsuitable). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
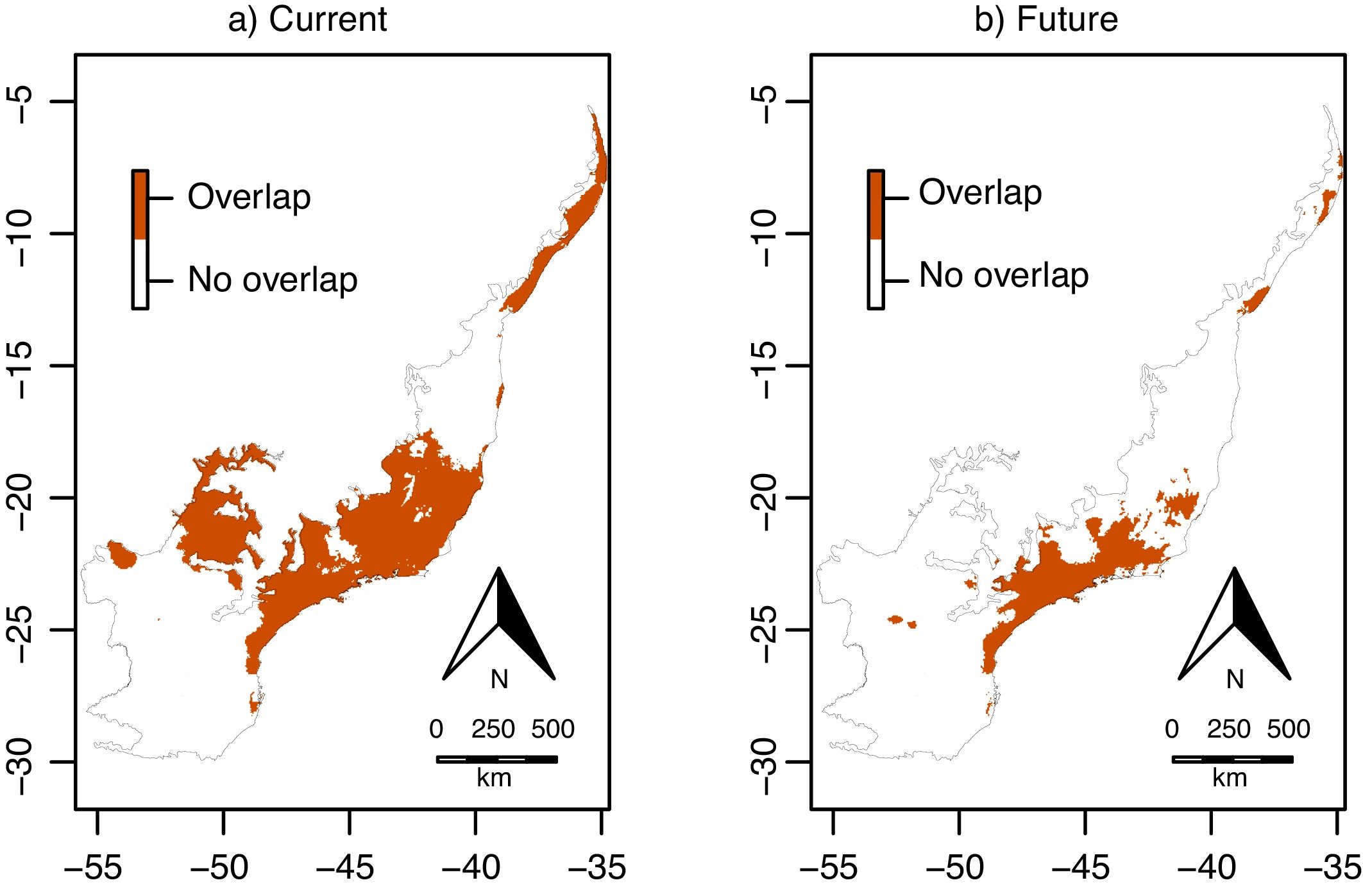
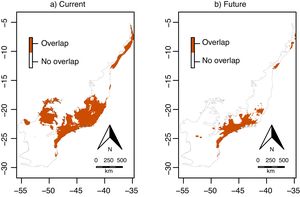
Climate change was estimated to decrease significantly in 65% the overlap area (i.e. the area suitable for both species simultaneously) across the Atlantic Forest, from 48,156,525ha (current) to 16,904,700ha (in 2070; χ=14.37, df=1, p<0.001; Fig. 6). These areas of overlap correspond to 46% and 29% of the current and future environmentally suitable areas for E. edulis (estimated at 103,675,950 and 58,644,000ha, respectively, as mentioned above). In both current and future climatic conditions, overlap was higher in the southeastern and northeastern portions of the Atlantic Forest, and lower in the southern region (Fig. 6), which was mostly unsuitable to E. oleracea (see Fig. 4a, b).
Overlap between the environmentally suitable areas of Euterpe edulis and Euterpe oleracea across the Atlantic Forest. Orange pixels are climatically suitable for both species under current (a) or future (2070; b) climatic conditions. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Our results suggest that the introduction of E. oleracea in the Atlantic Forest, combined with climate change, represent important additional threats to the already Vulnerable native palm E. edulis. Our overlap analyses indicate that around one-half and one-third of the environmentally suitable area of E. edulis is also suitable for the occurrence of E. oleracea, under current and future climatic conditions, respectively. Our results also show that climate change may affect congeneric native and non-native species differently, with negative impacts detected only for the native E. edulis. Finally, our analyses show that climate change impacts on E. edulis will be spatially heterogeneous across the Atlantic Forest. Taken together, our results highlight that the introduction of E. oleracea in the Atlantic Forest, combined with climate change, may lead to further decreases in the distributional range size of E. edulis. These findings are especially worrisome considering that E. edulis has a high ecological and economic importance in the Atlantic Forest (Galetti et al., 1999; Silva-Matos et al., 1999), and it is already threatened by palm heart harvest and deforestation (Martinelli and Moraes, 2013; Souza and Prevedello, 2019, 2020).
Previous studies have shown that climate change can decrease, increase or cause no changes in the distributional range size of native plant species (Bakkenes et al., 2002; Zwiener et al., 2018). In our study, we find that climate change is likely to decrease significantly E. edulis environmental suitability, especially at seasonal and dense forests. This decrease is probably related to the low tolerance of E. edulis to dry conditions, since climate change will increase temperatures and decrease precipitation at these physiognomies (see Figs. S2 and S3), that might result in a soil water availability at levels below E. edulis physiological tolerance (Junior et al., 2003; Gatti et al., 2014). In contrast, environmental suitability for E. edulis was predicted to slightly increase in the south portion of the Atlantic Forest, which is dominated by mixed forest. This increase is probably related to the low tolerance of E. edulis to lower temperatures (Junior et al., 2003; Gatti et al., 2008), as climate change will bring warmer (and also wetter) conditions in the currently colder southern portion of the Atlantic Forest (see Figs. S2 and S3).
Previous studies have found contrasting results of how climate change will alter the potential distribution of non-native species (Bradley et al., 2010; Bezeng et al., 2017). Our results show that predicted changes in temperature and precipitation will not change significantly environmentally suitability for E. oleracea in the Atlantic Forest, evidencing that the invasion risk may be similar in current and future scenarios. In contrast to our results, Vaz and Nabout (2016) showed that climate change may result in a small increase in distributional area of E. oleracea along the Amazon region. Our models indicate that E. oleracea has the potential to occur in a significant area of the Atlantic Forest, especially in the southeastern region, under both current and future climatic conditions (see Fig. 5). On the other hand, the mixed forest of the southern region appears to be unsuitable to this species, in marked contrast to the native E. edulis. The low suitability of the southern region to E. oleracea was expected considering its original distribution, which is concentrated mostly in the much warmer Amazon region (Henderson et al., 1995), suggesting that E. oleracea has a lower tolerance to colder temperatures, compared to E. edulis. Importantly, the mixed forest is likely to remain unsuitable for E. oleracea in the future (see Fig. 5b), despite the future warming predicted for southern Brazil.
Our environmental suitability models predicted that around 46% of the current environmentally suitable area for E. edulis is also suitable for its congeneric E. oleracea. Despite climate change may reduce the overall extent of overlap, due to the significant reduction in environmentally suitable area for E. edulis, the future overlap area will still encompass almost one-third of the remaining environmentally suitable area for E. edulis, which will be about 40% smaller than the current suitable area. These results evidence the high invasion potential of E. oleracea in areas occupied by E. edulis, especially in the southeastern region of Brazil, where the extent of overlap is higher under both current and future climatic conditions (see Fig. 6). Such high overlap in the southeastern region is worrisome, considering that the introduction of E. oleracea in the Atlantic Forest started in this region in the 1970s (Bovi et al., 1987; Tiberio et al., 2016). Even with the subsequent discontinuity of planting activities in the region, it was reported that E. oleracea individuals resprouted after cutting. This resprouting ability, due to clonal growth, can make the management of E. oleracea even more problematic, since plant death only occurs when the whole plant (genet and their associated ramets) is killed (Pyšek, 1997). Clonal growth is a common trait in invasive plant species (Song et al., 2013), which may increase their invasiveness by increasing drought and disturbance resistance, potentially representing a competitive advantage over native species (Liu et al., 2016). In addition, the presence of spontaneous hybrids of E. edulis and E. oleracea has already been reported (Tiberio et al., 2016), which may favor the non-native in detriment of the native species due to genetic and demographic reasons. Introgressive hybridization can result in the replacement of native individuals by hybrids of superior fitness, a process referred to as genetic swamping (Wolf et al., 2001). Also, hybridization can increase the invasiveness by providing sufficient mates for the non-native species to overcome Allee effects (Mesgaran et al., 2016). On the other hand, the introduction of exotic alleles through hybridization can disrupt the process of local adaptation of native species, by extirpating adapted genes, increasing species’ risk of extinction (Rhymer and Simberloff, 1996; Prentis et al., 2007). In addition, hybridization can cause demographic swamping, that is, when population growth rate of native species is below replacement due to wasted reproductive effort on infertile hybrids (Wolf et al., 2001).
Similarly to most environmental suitability models (Peterson et al., 2011), our models have two main limitations in predicting environmental suitability for both native and non-native species. First, our analyses were restricted to climate and elevation variables, whereas other intrinsic and extrinsic factors could prevent the occupation of a seemingly suitable area by both species, such as soil type, interspecific interactions, habitat fragmentation and limited dispersal ability (Thuiller et al., 2005; Jimenez-Valverde et al., 2011). Secondly, the available occurrence records may not represent the full range of environmental conditions tolerated or required by species, due to dispersal limitation and restricted survey effort, for example (Peterson et al., 2011). Despite these limitations, however, our analyses allowed to detect general, clear and robust patterns in terms of invasion potential and climate change impacts on E. edulis and E. oleracea.
In conclusion, our models show that both the introduction of the congeneric E. oleracea and climate change represent two additional potential threats to E. edulis. The combined effects of climate change and biological invasion could further increase the extinction risk of the already threatened E. edulis, since most of their populations are currently impoverished due to palm heart overharvesting and deforestation (Silva-Matos et al., 1999; Souza and Prevedello, 2019, 2020). Therefore, we discourage the introduction of E. oleracea in the Atlantic Forest, even for economic activities that intend to decrease harvest pressure on E. edulis, as E. oleracea may spread to natural areas outside plantations (Tiberio et al., 2016). We recommend avoiding new introductions and monitoring already introduced individuals especially in the southeastern portion of the Atlantic Forest, which is environmentally suitable to both species under current and future scenarios. In addition, we recommend maintaining and expanding protected areas especially in the southern region of the Atlantic Forest, where climatic suitability is predicted to increase for E. edulis and decrease for E. oleracea with climate change.
This study was supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (processes E-26/010.002334/2016 and E-26/ 010.000398/2016) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; process 424061/2016-3).
The following are the supplementary data to this article:
Change in annual precipitation (a), temperature annual range (b) and mean temperature of warmest quarter (c) across the Atlantic Forest. Change values were calculated as the difference between future and current climatic values. In (a), positive values indicate increases in precipitation (in blue scale). In (b), positive values indicate increases in temperature range (in red scale). In (c), positive values indicate increases in mean temperature (in red scale).