Natural fires are an important ecological process that over millennia have shaped savannahs. Local mammalian assemblage structure is influenced by vegetation diversity and complexity, whereby changes in composition and structure of vegetation due to fire absence lead to the reorganization of small-bodied mammal assemblages. We aimed to evaluate how small-bodied mammal assemblages respond to prolonged (i.e., 17 years) fire absence, given that this issue is crucial to understanding the long-term reassembling of small-bodied mammal assemblages in the Cerrado. In our study at Serra das Araras Ecological Station (SAES), we compared small-mammal assemblages sampled in 2016–2017 — during a prolonged period without fire — with those from a study conducted in 1999–2000, when fires had been occurring naturally. We used descriptive statistics, rarefaction to assess species richness, and Non-Metric Multidimensional Scaling (NMDS) with SIMPROF for site-specific analysis of mammal composition between both periods. Our major result showed clearly that the absence of natural fires during 2001–2016 in the SAES reassembled the small-bodied mammal fauna compared to the period with constant fires (1995–2000). Our findings indicate that most species are common to both forest and savannah environments, reflecting the homogenization of habitats due to the absence of natural fires. Based on our study, we can conclude that the absence of fire has partially reassembled the small-bodied mammal assemblages across an important protected area of Brazilian Cerrado. Considering that fire is a crucial factor for the dynamics of the Cerrado — which has evolved historically under fire-driven processes — further technical discussions about fire management are needed given its crucial role in maintaining (or erasing) aspects of local diversity, especially with high stocks of dry biomass. In terms of conservation, the evidence so far showed that big-fires are conclusively disastrous, but the absence of natural fires in native areas of Cerrado apparently also harmed the Cerrado-prone biotas.
Natural fires are an important ecological process that over millennia have shaped tropical savannahs, such as Brazilian Cerrado (Fox, 1982; Kreider et al., 2023). The Cerrado marks the final phase of the transition from eutrophic tropical seasonal forests to a pedobiome and pyrobiome dominated by tropical moist savannahs and grasslands (Coutinho, 1990). The Cerrado is not a uniform ecosystem. It spans a diverse ecological gradient, including the cerradão (a dense, scleromorphic forest), cerrado sensu stricto (typical cerrado), campo cerrado (a shrubby savannah), campo sujo (a sparse savannah), and campo limpo (a dystrophic grassland). These savannah formations can be seen as broad ecotones between the extremes of cerradão and campo limpo. Moreover, the Cerrado biome is characterized by a remarkable presence of riparian forests (gallery forests), habitats with a high density of palms (e.g., Babaçu [Attalea speciosa] forests), and the presence of dry-deciduous forests (Coutinho, 1990). In a broad sense, the Cerrado biome can be considered part of a large ecocline that spans Brazil, primarily shaped by gradients in soil fertility and fire incidence (Coutinho, 1990; Durigan, 2020).
Both the absence of fires and the occurrence of megafires have chronically reshaped the Cerrado biome, as fires of different intensities promote the reassembly of biological communities. Natural fire regimes are essential to maintaining ecological balance, yet there is limited understanding of the ideal fire patterns and frequencies for the diverse Cerrado phytophysiognomies, which have evolved to be fire-adapted (Alves and Silva, 2011). In the absence of fire, the density of woody plants increases in many areas of the Cerrado, leading to the encroachment of woody species. This phenomenon is generally observed in savannahs — including the Cerrado — due to the key role of fire in limiting the growth of woody plants. In fire-prone environments, frequent fires reduce the accumulation of biomass and prevent the establishment and expansion of woody species. When fire is suppressed, woody plants can outcompete grasslands, leading to an increase in tree and shrub cover (Stevens et al., 2016; Maracahipes-Santos et al., 2018; Rosan et al., 2019; Eldridge and Ding, 2021).
Fire suppression in Cerrado protected areas has led to a biodiversity shift across multiple levels. At the landscape level, it has homogenized vegetation structure; at the species level, it has driven local extinctions, particularly among non-tree species; and at the population level, it has likely impaired sexual reproduction, further threatening ecosystem diversity (Durigan, 2020). According to Federal Law No. 11,516 (Brasil, 2007), the fire suppression management strategy includes fire prevention through environmental zoning, the implementation of firebreaks and physical barriers, prescribed burns, climatic monitoring, and the deployment of personnel for fire control. Megafires, however, have ravaged Brazilian biomes. In Brazil, extensive areas of the Cerrado have burned, reigniting the debate over the "zero-fire" policy. Many protected areas in the biome have implemented strict fire exclusion and prevention strategies, which have paradoxically contributed to periodic megafire events (Fidelis et al., 2018), whereas megafires can have devastating effects on biodiversity (Geary et al., 2021).
The Brazilian Cerrado is a hotspot of biodiversity. In Brazil, Cerrado is the second biome with the most threatened species (ICMBio, 2018), primarily due to habitat loss, megafires, and fragmentation driven by the rapid agribusiness expansion (ICMBio, 2018). The Cerrado biome embraces 118 small-bodied mammal species, including rodents and marsupials typically ≤1 kg (Mendonça et al., 2018). However, the evidence so far indicates that local mammalian assemblage structure is influenced by vegetation diversity and complexity, whereby changes in composition and structure of vegetation due to fire absence lead to the reorganization of small-bodied mammal assemblages (Alho et al., 1986; Carmignotto et al., 2012, 2014). In the Cerrado, for instance, fire promotes herbaceous cover dominance, while fire exclusion allows woody vegetation to thrive (Hoffmann, 1999; Moreira, 2000; Hoffmann and Moreira, 2002). Such ecological shifts affect mammal species composition and abundance, as savannahs invaded by shrubs tend to negatively impact species that prefer open environments, while generalist and forest-adapted species may benefit (Stanton et al., 2017; Furtado et al., 2021).
Small-bodied non-volant mammals (rodents and marsupials) in the Cerrado are particularly vulnerable to habitat changes due to their high habitat selectivity and low dispersal capacity (Pardini et al., 2010; Gutiérrez and Marinho-Filho, 2017; Carmignotto, 2019). Protected areas, such as the Serra das Araras Ecological Station (SAES) have a vital role in supporting a rich diversity of mammals (Santos-Filho et al., 2012). In accordance with Federal Law No. 11,516 (Brasil, 2007), the SAES has prioritized wildfire prevention through suppression strategies, including resource and personnel allocation during the dry season. However, while these measures aim to control uncontrolled wildfires, prescribed burning was not implemented under favorable conditions during this period. As a result, fire has been nearly eliminated from the region’s ecosystems, despite the ecological importance of managed fire regimes. Thus, we aimed to evaluate how small-bodied mammal assemblages respond to prolonged (i.e., 17 years) fire absence, given that this issue is crucial to understanding the long-term reassembling of small-bodied mammal assemblages in the Cerrado. We hypothesized that fire suppression at the Serra das Araras Ecological Station (SAES) has led to changes in both small-bodied mammal richness, occurrences and composition, in a process of turnover that favour forest species to the detriment of the savannah-adapted species.
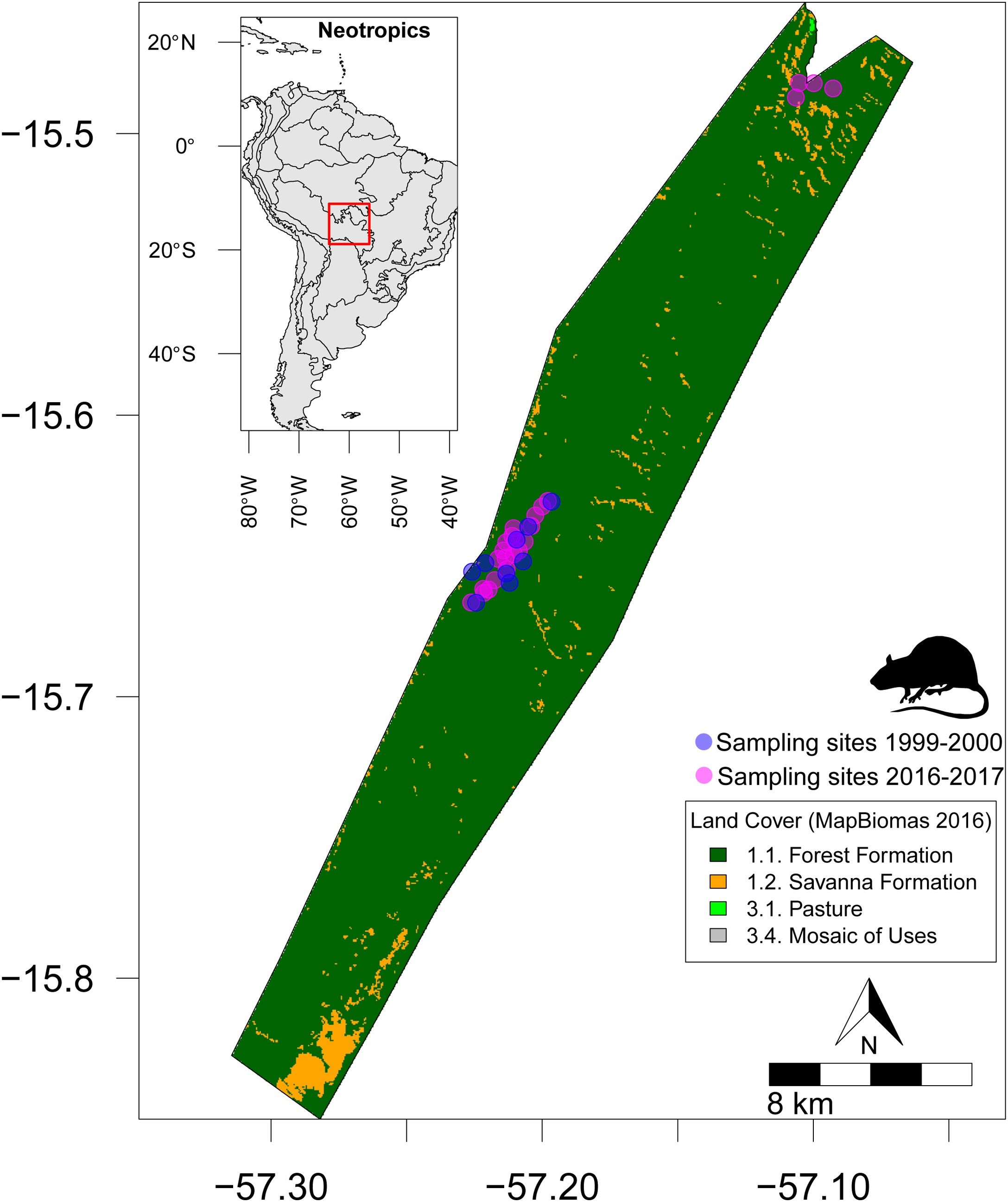
Material and methodsStudy areaOur study was developed at Serra das Araras Ecological Station (SAES) (Fig. 1), a protected area located in the southwestern region of Mato Grosso state, Brazil. Situated in the Província Serrana across the Upper Paraguay basin (Franco and Pinheiro, 1982; ICMBio, 2016) between the parallels 15º27’-15º48’S and 57º1’-57º2’W, SAES has a range elevation from 300 to 800 meters and encompasses 28,700 hectares (Brasil, 1982). SAES stays in the Cerrado morphoclimatic domain, across a corridor formed by parallel mountains (ICMBio, 2016). This protected area is covered by several phytophysiognomies, including cerrado stricto sensu (hereafter cerrado ss), gallery forests (hereafter gallery forest), babaçu forests (hereafter babaçu), and dry-deciduous forests (hereafter dry forests) (Ribeiro et al., 2008). According to the Köppen classification, the climate in the region is categorized as Aw (i.e., tropical savannah conditions with distinct wet and dry seasons). The average annual temperature ranges from 24 °C to 26 °C, with the rainy season occurring between October and April, and the dry season from May to September. Annual precipitation in the SAES varies from 1,300 to 1,600 mm (Alvares et al., 2014).
Location of small-bodied mammal sampling sites across Serra das Araras Ecological Station (SAES), Brazilian Cerrado. Cyan points represent sampling sites in 1999-2000 period (i.e., fire presence), whereas magenta points represent 2016–2017 period (i.e., fire absence). Land cover was obtained from MapBiomas project (https://brasil.mapbiomas.org/).
In 2016 and 2017 (when natural fires were absent due to management; Federal Law No. 11,516 (Brasil, 2007)), we collected small-bodied mammals across 24 independent sites representing four distinct Cerrado phytophysiognomies: 8 sites in the cerrado ss, 6 sites in gallery forest, and 6 sites in babaçu, and 4 sites in dry forests. We prior selected these sites using the Landsat 8 (2016) satellite imagery. For small-bodied mammals sampling we used conventional Sherman (80 × 90 × 230 mm) and Tomahawk (145 × 145 × 410 mm) traps. In doing so, at each site, three parallel transects, each approximately 200 meters long and spaced 50 meters apart, were established. Each transect featured 20 capture stations spaced 10 meters apart. In gallery forest, babaçu, and dry forest, each capture station was equipped with either a Sherman or Tomahawk trap, placed either on the ground or in the understory at approximately 2 meters in height, alternating positions to balance the number of traps at both levels. In the cerrado ss, all traps were set on the ground. The traps were baited with banana and peanut butter and checked daily over a period of 10 consecutive nights, except in dry forest sites, where trapping occurred for eight days. During the nine months of fieldwork (i.e., dry season: September-2016 and from May to June-2017; rainy season: from October-2016 to March-2017), a total sampling effort of 13,960 trap-nights was performed, whereas capture success was calculated using the following equation: capture success = (captures + recaptures/sampling effort) × 100. For each species, the first 10 individuals captured at each site were placed in cotton cloth bags and transported to the SAES support base, where they were euthanized, measured, weighed, examined for reproductive status, and taxidermized. Any additional captured individuals, after species identification and biometrics, were tagged with sequentially numbered tags (National Band & Tag Co.) and released at the capture site. This study was initiated before the establishment of an Animal Ethics Committee (CEUA) at our institution. Thus, all procedures involving animals were performed following the ethical guidelines and regulations preconized to the American Society of Mammalogists guidelines for animal care (Sikes, 2016) and were conducted under SISBIO permit number 54897-1.
Specimens were prepared following standard scientific collection protocols and deposited in the mammal collection of the Universidade do Estado de Mato Grosso (UNEMAT) in Cáceres-MT, Brazil. Species identification was based on taxonomic identification keys from Bonvicino et al. (2008); Gardner (2008); Reis et al. (2011), and Patton et al. (2015) and further confirmed by consulting the Mammal Collections at UNEMAT and the Federal University of Mato Grosso (UFMT). Our dataset was therefore compared with a prior study conducted by Santos-Filho et al. (2012) in the SAES. From May 1999 to January 2000 (when natural fires were present in the region; Miranda et al., 2002), Santos-Filho et al. (2012) sampled four Cerrado phytophysiognomies, covering 3 sites in cerrado ss, 3 sites in babaçu, 3 sites in gallery forest, and 2 sites in rupestrian fields. The sites, trap arrangements, sampling effort and seasons were similar to those in our study (see Santos-Filho et al., 2012), resulting in a total sampling effort of 13,200 trap-night. Data from rupestrian fields and dry forests were excluded from comparisons between 1999–2000 and 2016–2017 due to insufficient sampling alignment: no rupestrian field sites were sampled in 2016–2017 (reducing the total to 9 sites), and dry forests lacked replicas in 1999–2000, but were maintained in descriptive statistics of our study.
Fire amount during both periodsUsing the Google Engine platform (https://code.earthengine.google.com/) based on the MapBiomas Collection 3.0 fire database (2024) we extracted the total area burned from 1995 to 2000, representing the fire amount prior to Santos-Filho et al. (2012) sampling (i.e., during the presence of natural fires) and from 2001 to 2016, representing the fire amount prior to our study but posterior to Santos-Filho et al. (2012). In doing so, we established a radial area of 500 m (∼78 ha) around the geocoordinates of any site expressed in decimal degree (SIRGAS 2000) sampled during 1999−2000 or 2016−2017 and, therefore, obtained the total amount of burned area (ha) for each site in both periods (Ntotal = 20 + 9 = 29).
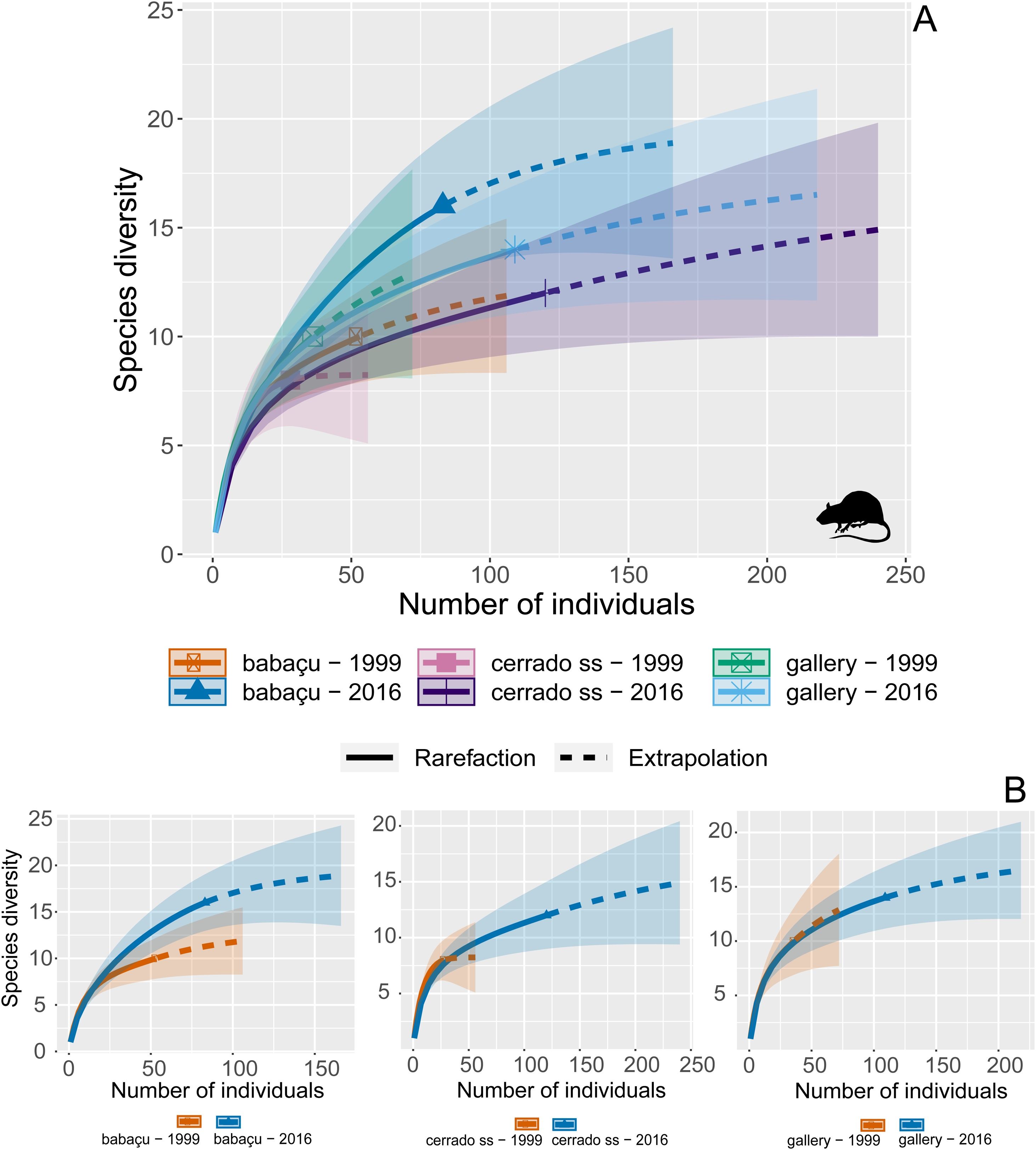
Data analysisWe first explored the data based on descriptive statistics (e.g., species richness, occurrence, and abundance) and employed a rarefaction approach to both evaluate the sampling effort and compare the species richness per phytophysiognomy (i.e., amounting the species abundance of each site according to its phytophysiognomy). To do so, we followed the method described by Chao et al. (2014), which estimates species richness based on equivalent sample coverage across all sites. Sample coverage, reflecting inventory completeness (i.e., considering sampling coverage acceptable typically ≥75%), uses richness values for a given sample size or coverage, enabling statistical comparisons in the 95% confidence intervals around the interpolated-extrapolated rarefaction curves. These comparisons are typically based on twice the minimum abundance of any assemblage (Chao et al., 2014; Hsieh et al., 2019) and we implemented this analysis using the iNEXT R-package (Hsieh et al., 2019) in an R code (R Core Team, 2024).
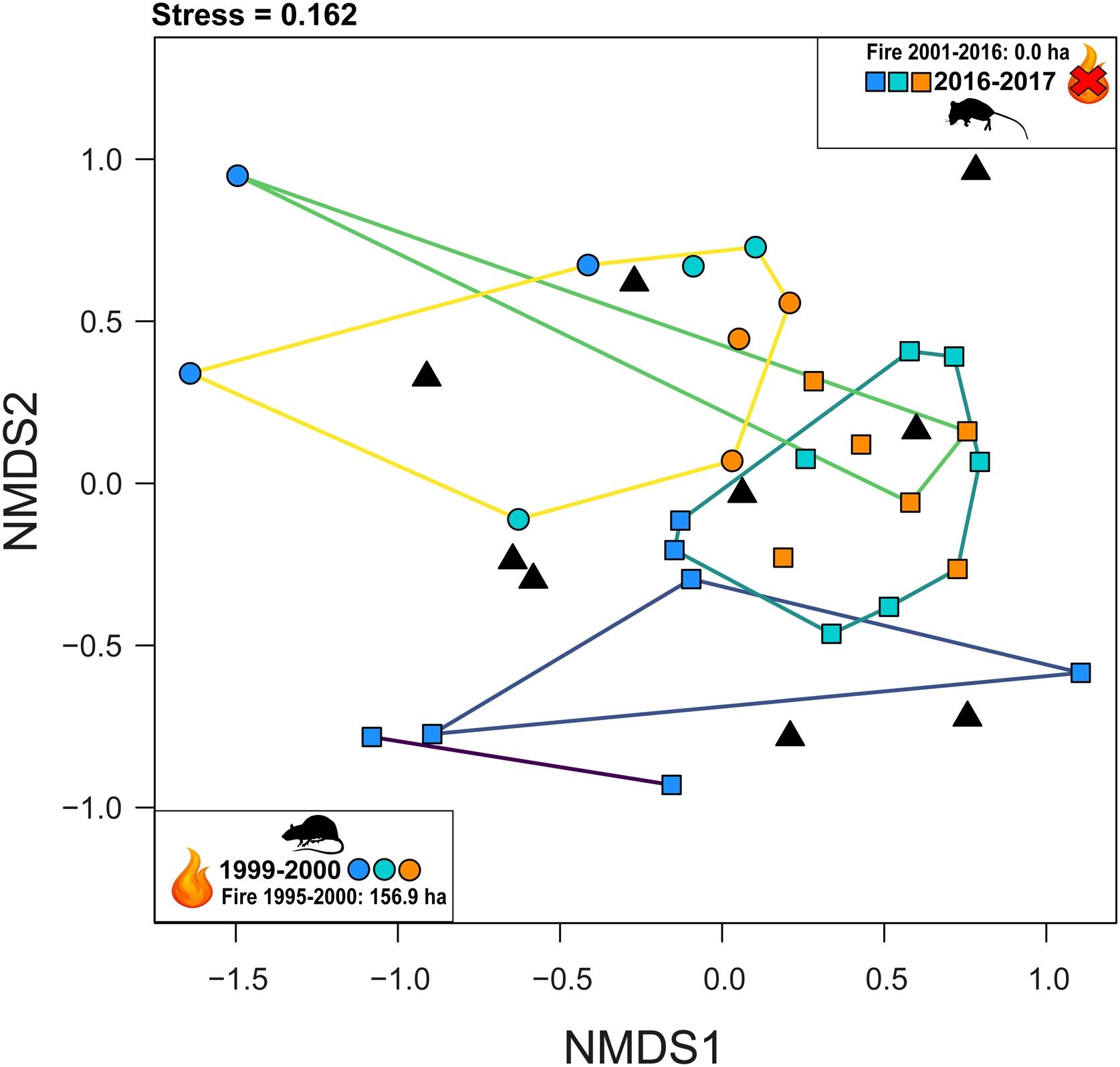
To evaluate the small-bodied mammal compositions at each site- phytophysiognomy and period (i.e., 2016−2017 and 1999−2000), focusing on habitat-type specificity, we performed an ordination of sites using Non-Metric Multidimensional Scaling (NMDS) analysis (Mead, 1992) using the Bray-Curtis (1957) distance coefficient. A key output of NMDS is the stress value, which represents the degree of mismatch between the similarity matrix and the graphical arrangement of the axes (Clarke and Warwick, 2001). Further, we created a null dataset of species composition to include in the NMDS analysis. In doing so, we randomized the species abundance ranging from 0 (min.) to 18 (max. abundance) in the adjacency matrix i (i.e., columns representing the total of species during both periods; N = 27) across 9 theoretical sites j (i.e., Nmin. of sites in Santos-Filho et al., 2012), but forcing the randomization across to i,j to maintaining the proportion of absences (i.e., zeros) in the datasets (i.e., ∼70%). Finally, we also evaluated the significance of NMDS analysis using a Similarity Profile Analysis (SIMPROF), with groupings considered statistically significant at p ≤ 0.05. SIMPROF identifies the number of significant clusters within a dendrogram or ordination (Clarke et al. 2008). We conducted these analyses in R (R Core Team, 2024) based on clustsig (Whitaker and Christman, 2010) and pvclust R-package (Suzuki et al., 2019).
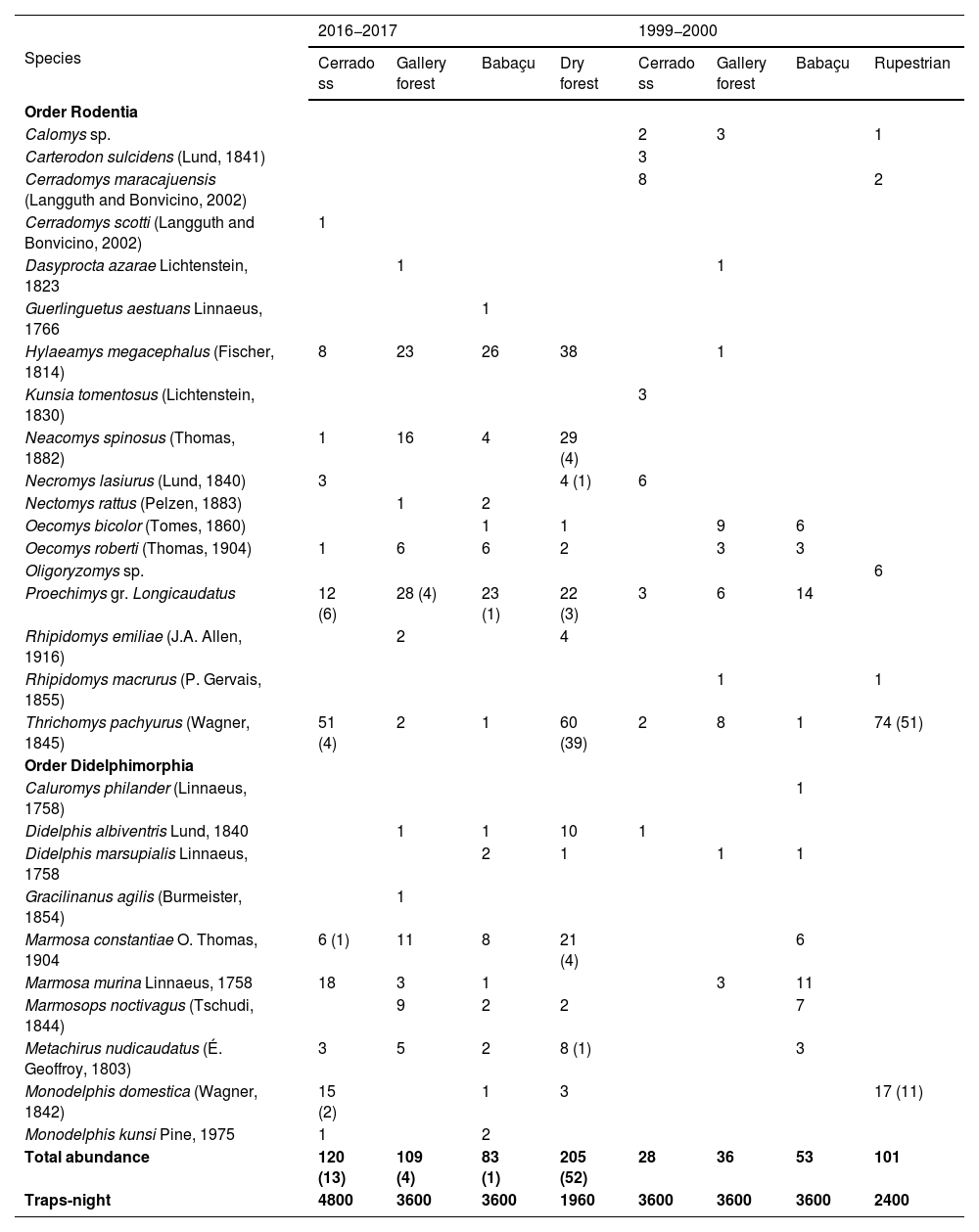
ResultsIn 2016−2017 we performed 587 captures, including 70 recaptures, yielding a capture success rate of 4.20%. Our effort in 2016−2017 led to a total richness of 21 small-bodied mammal species, including 12 rodent species and 9 marsupial species. Among the rodents, Thrichomys pachyurus had the highest number of both captures (N = 114) and recaptures (N = 43), while the marsupial Marmosa constantiae had 46 captures and 5 recaptures, with individuals recorded across all four sampled phytophysiognomies. Our results also showed that some species were restricted to only one phytophysiognomy: Cerradomys scotti was exclusive to cerrado ss, Guerlinguetus aestuans to the babaçu forest, and both Dasyprocta azarae and Gracilinanus agilis in the galleria (Table 1). Moreover, during 2016−2017 species richness varied between phytophysiognomies, being 1–6 species in cerrado ss, 3–12 in babaçu sites, 3–8 in gallery forest (9–12 species in the dry forest, not considered in statistical analysis).
Small-bodied mammal species richness per different phytophysiognomies and periods across Serra das Araras Ecological Station (SAES), Brazilian Cerrado. The values in parentheses represent species recaptures, whereas dry forests and rupestrian filed was not used for data comparisons due to absence of replicas between the distinct periods. Data from 1999 to 2000 was obtained in Santos-Filho et al. (2012).
| Species | 2016−2017 | 1999−2000 | ||||||
|---|---|---|---|---|---|---|---|---|
| Cerrado ss | Gallery forest | Babaçu | Dry forest | Cerrado ss | Gallery forest | Babaçu | Rupestrian | |
| Order Rodentia | ||||||||
| Calomys sp. | 2 | 3 | 1 | |||||
| Carterodon sulcidens (Lund, 1841) | 3 | |||||||
| Cerradomys maracajuensis (Langguth and Bonvicino, 2002) | 8 | 2 | ||||||
| Cerradomys scotti (Langguth and Bonvicino, 2002) | 1 | |||||||
| Dasyprocta azarae Lichtenstein, 1823 | 1 | 1 | ||||||
| Guerlinguetus aestuans Linnaeus, 1766 | 1 | |||||||
| Hylaeamys megacephalus (Fischer, 1814) | 8 | 23 | 26 | 38 | 1 | |||
| Kunsia tomentosus (Lichtenstein, 1830) | 3 | |||||||
| Neacomys spinosus (Thomas, 1882) | 1 | 16 | 4 | 29 (4) | ||||
| Necromys lasiurus (Lund, 1840) | 3 | 4 (1) | 6 | |||||
| Nectomys rattus (Pelzen, 1883) | 1 | 2 | ||||||
| Oecomys bicolor (Tomes, 1860) | 1 | 1 | 9 | 6 | ||||
| Oecomys roberti (Thomas, 1904) | 1 | 6 | 6 | 2 | 3 | 3 | ||
| Oligoryzomys sp. | 6 | |||||||
| Proechimys gr. Longicaudatus | 12 (6) | 28 (4) | 23 (1) | 22 (3) | 3 | 6 | 14 | |
| Rhipidomys emiliae (J.A. Allen, 1916) | 2 | 4 | ||||||
| Rhipidomys macrurus (P. Gervais, 1855) | 1 | 1 | ||||||
| Thrichomys pachyurus (Wagner, 1845) | 51 (4) | 2 | 1 | 60 (39) | 2 | 8 | 1 | 74 (51) |
| Order Didelphimorphia | ||||||||
| Caluromys philander (Linnaeus, 1758) | 1 | |||||||
| Didelphis albiventris Lund, 1840 | 1 | 1 | 10 | 1 | ||||
| Didelphis marsupialis Linnaeus, 1758 | 2 | 1 | 1 | 1 | ||||
| Gracilinanus agilis (Burmeister, 1854) | 1 | |||||||
| Marmosa constantiae O. Thomas, 1904 | 6 (1) | 11 | 8 | 21 (4) | 6 | |||
| Marmosa murina Linnaeus, 1758 | 18 | 3 | 1 | 3 | 11 | |||
| Marmosops noctivagus (Tschudi, 1844) | 9 | 2 | 2 | 7 | ||||
| Metachirus nudicaudatus (É. Geoffroy, 1803) | 3 | 5 | 2 | 8 (1) | 3 | |||
| Monodelphis domestica (Wagner, 1842) | 15 (2) | 1 | 3 | 17 (11) | ||||
| Monodelphis kunsi Pine, 1975 | 1 | 2 | ||||||
| Total abundance | 120 (13) | 109 (4) | 83 (1) | 205 (52) | 28 | 36 | 53 | 101 |
| Traps-night | 4800 | 3600 | 3600 | 1960 | 3600 | 3600 | 3600 | 2400 |
In 1999−2000 were made 280 captures (62 recaptures) in a capture success rate of 1.7%, whereby 21 taxa: 13 rodents, and 8 belonging to the order Didelphimorphia were recorded (Table 1). Disregarding rupestrian fields, the most abundant (N ≥ 10) species in this period were Oecomys bicolor, Proechimys gr. longicaudatus, Thrichomys pachyurus, and Marmosa murina (Table 1; Supporting Information S1) were mainly or exclusively recorded in gallery forest and babaçu environments (Table 1). The taxon exclusive to 2016−2017 were Cerradomys scotti, Neacomys spinosus, Nectomys rattus, Rhipidomys emiliae, Guerlinguetus aestuans, Gracilinanus agilis, and Monodelphis kunsi, whereas the taxon exclusive to 1999−2000 were Rhipidomys macrurus, Kunsia tomentosus, Carterodon sulcidens, Cerradomys maracajuensis, Calomys sp., and Caluromys philander (Table 1).
Comparing the sampling coverage and species richness between both phytophysiognomies and periods we found a sampling coverage ranging from 89% to 97%, whereas no statistical difference phytophysiognomies-periods was detected (Fig. 2), given that the species richness estimates ranged from 8.24 (cerrado ss – 1999−2000) to 19.6 (babaçu – 2016−2017) but with confidence intervals overlapping in the twice-abundance extrapolation (Fig. 2). However, when we compared the species composition we found a clear difference in species occurrence in 1999−2000 vs. 2016−2017. Our NMDS (Stress = 0.162) followed by SIMPROF analysis revealed 5 distinct significant clusters, clearly separating phytophysiognomies in both periods (Fig. 3), whereas the NMDS (Stress = 0.100) for null matrix resulted in an ordination totally at random and pan-distributed across the ordination space (Fig. 3). Finally, during the period of 1995–2000, 156.9 ha (6.1%) of sampling sites buffers (amounting 2,574 ha) were burned, whereas from 2001 to 2016 no fire was recorded in those sites (Fig. 3).
(A) Rarefaction analysis to evaluate the sampling effort and compare the small-bodied mammal species richness per different phytophysiognomies and periods. (B) Rarefaction analysis to evaluate the sampling effort and compare the small-bodied mammal species richness in each phytophysiognomies separately by periods across Serra das Araras Ecological Station (SAES), Brazilian Cerrado.
Non-Metric Multidimensional Scaling (NMDS) ordination of small-bodied mammal compositions across sites and periods (1999–2000 and 2016–2017), using Bray-Curtis distance. Stress value indicates goodness-of-fit between similarity matrix and ordination. Clusters were validated with Similarity Profile Analysis (SIMPROF), identifying significant groupings (p ≤ 0.05). Null models represent the species abundance randomized ranging from 0 (min.) to 18 (max. abundance) in the adjacency matrix i (i.e., columns representing the total of species during both periods). Circles represents data from 1999 to 2000, squares data from 2016-2017, and triangles data from null models. In any period, orange represents babaçu forests, blue cerrado ss, turquoise represents gallery forests, whereas black triangles represents null models. The lines represent five distinct significant grouping and different color in the lines is only to distinction.
Understanding how species diversity, distribution, and abundance shift spatiotemporally is a major challenge in ecology and conservation, especially when it comes to the effects of fire. Our major result showed clearly that the absence of natural fires during 2001–2016 in the SAES reassembled the small-bodied mammal fauna compared to the period with constant fires (1995–2000). Moreover, the absence of statistical difference in species richness between phytophysiognomies and periods reinforces that the fires — even at low rates — can reassemble small-bodied mammal assemblages without significant changes in species richness, resulting in a species turnover process and increasing the beta-diversity between these periods (Koleff et al., 2003). Different from species richness, the turnover rates are responsible for depicting changes in species composition spatiotemporally, where some species are replaced by others generally better adapted to new habitat conditions or competitive forces (Hillebrand et al., 2017; O’Sullivan et al., 2021).
Our results suggest — at least partially — a replacement of species typically of savannah by forestry species. Among 6 species captured in 1999−2000 that were not present in 2016−2017, 4 (66.7%) are rare species from savannah habitats: Carterodon sulcidens, Kunsia tomentosus, and Calomys sp. (Santos-Filho et al., 2012). Despite this pattern, we acknowledge that these aforementioned species are naturally rare throughout its distribution range (e.g., Bezerra and Pardiñas, 2016), precluding a complete overview about the species replacement. In contrast, among 7 species recorded only in 2016−2017, 6 (85.7%) are generalist or primarily associated with forest habitats, such as Neacomys spinosus and Nectomys rattus (Carmignotto et al., 2014; Carmignotto, 2019), but were species with a few records in the SAES. Only one individual of Cerradomys scotti — a species typical of savannah habitats — was collected in the cerrado ss during 2016−2017, although it is less frequently found in “veredas”, riparian forests, and “cerradão” (Langguth and Bonvicino, 2002; Furtado et al., 2021). Notably, only one individual of Hylaeamys megacephalus was collected in 1999−2000 from the gallery forest, while 95 individuals were collected in 2016−2017, including 8 from the cerrado ss. This species inhabits a variety of biomes but is always restricted to forest habitats (Mares et al., 1986; Ochoa et al., 1993), whereas Neacomys spinosus was not recorded previously, but in 2016−2017, 50 individuals were captured, given that the species is often found in primary and secondary forests (Bonvicino et al., 1996; Patton et al., 2000).
These changes in species composition are associated with the restructuration of Cerrado habitats, similar to the changes caused by deforestation of the Amazon (e.g., Santos-Filho et al., 2024). The Cerrado is a biome with species adapted to natural fires, but fire exclusion led to a change in the phytodemographic dynamics, given that some species previously in low abundance or absent became dominant changing the savannic habitat to a forested habitat (Simon et al., 2009). Consequently, the faunas associated with these different habitats tend to change. For instance, from invertebrates (e.g., Costa et al., 2022) to vertebrates (e.g., Manica et al., 2010) important changes in species composition are derived from forest vs. savannah environments. For small-bodied mammals, fire-mediated differences also are affected by species traits (Culhane et al., 2022) whereas vegetation attributes have a fundamental role in the occupancy by small mammals (González et al., 2021). Despite no changes in species richness, the greater complexity of the forest phytophysiognomy — which offers more vertical structure and resource availability — in theory could support higher species diversity (August, 1983; Tews et al., 2004; Santos-Filho et al., 2012). This subject deserves further investigation. Numerous studies have established a positive relationship between small-bodied mammal richness and structural complexity of vegetation (August, 1983; Mendonça et al., 2018; Carmignotto, 2019; Carmignotto et al., 2022). Although the dissimilarity of species between forest and savannah environments in the Cerrado has been well-documented in previous studies (e.g., Mares et al., 1986; Bonvicino et al., 1996; Carmignotto and Aires, 2011; Carmignotto et al., 2014), our findings indicate that most small-bodied mammal species were common to both forest and savannah environments, being able to reflect an “in progress” habitat homogenization due to the absence of natural fires.
On the other hand, the acute presence of criminal wildfires has devastated several Brazilian biomes in recent years. In the past five years, Brazil has experienced unprecedented wildfires, burning 12.5% of its territory (MapBiomas, 2024). For example, the devastating 2020 wildfires in the Pantanal wetlands led to the loss of an estimated 17 million vertebrates across ∼39 km2 (Tomas et al., 2021). A recent study using camera-traps and environmental DNA revealed that post-2020 fire Pantanal areas now support 37 species of medium- to large-bodied mammals, a number higher than before the Pantanal megafires and presented important changes in species composition (Magioli et al., 2024). In contrast, for example, our study revealed a marked increase in the abundance of Hylaeamys megacephalus, a species typically found in forested areas, following environmental changes associated with the absence of natural fires. Additionally, species previously considered rare or absent, such as Neacomys spinosus, have become more common. The habitat experiencing the most significant transformations — and consequently requiring the most attention — are the cerrado sensu stricto, representing a physiognomy characterized by a mix of woody and herbaceous vegetation. These regions have seen an encroachment of woody species, leading to the exclusion of native understory grasses. This shift has created a more forest-like environment, unsuitable for open-area specialist species, thereby allowing the incursion of forest-specialist species.
Overall, our findings indicate that the absence of fire can restructure small-bodied mammal assemblages in the SAES, but one major question remains: What is the ideal frequency and intensity of fires in the Cerrado areas to maintain typical habitats and species? In the fire absence, there has been a continuous increase in tree density, replacing grasses and shrubs with larger woody plants (Stevens et al., 2016; Maracahipes-Santos et al., 2018; Furtado et al., 2021). The lack of fire can eliminate or diminish typical habitats of the Cerrado savannah and its specific resources, such as burrows and grass seeds which are the main ecological requirements of these species. The response of small-bodied mammals to these changes reflects varying preferences for habitat use (Emmons and Feer, 1997; Santos-Filho et al., 2012; Ribeiro et al., 2020), leading to a shift in species composition as savannah species are replaced by those that are favored in more forested environments. Species typically associated with the Cerrado — such as Kunsia tomentosus and Carterodon sulcidens — have disappeared from these areas with the transition from savannah to forest. However, these results deserve further investigation. In Australian savannahs, several threats can interact synergistically together with fire, impacting small-bodied mammal assemblages differently, and therefore compromising the recommendations for fire management against distinct threats to biodiversity (Legge et al., 2019).
To further address and understand these dynamics, we can recommend, for example, studies incorporating controlled fires, given that this phenomenon influences vegetation succession in three distinct ways: (1) an early succession group peaking within two years after the fire, mainly composed by native grasses and shrubs; (2) a second group of woody trees peaking between two and nine years; and (3) a late succession group of plants reaching its peak after 10 years post-fire (Vieira, 1999; Mendes-Oliveira et al., 2012; Holmes and Robinson, 2016; Lindenmayer et al., 2016). The diversity and abundance of small mammals tend to reach their maximum values in the initial stages of succession, and fire suppression may lead to population extinctions through habitat modification and disruption of demographic processes (Briani et al., 2004; Hutto, 2008; Templeton et al., 2011). Based on our study, we can conclude that the absence of fire has partially reassembled the small-bodied mammal assemblages across an important protected area of Brazilian Cerrado. Considering that fire is a crucial factor for the Cerrado dynamics — which has evolved historically under fire-driven processes — further technical discussions about fire management are needed given its crucial role in maintaining (or erasing) aspects of local biodiversity, especially in areas with high stocks of dry biomass. In terms of conservation, the evidence so far showed that big-fires are conclusively disastrous, but the absence of natural fires in native areas of the Cerrado also appears to have negatively affected fire-adapted biotas.
CRediT authorship contribution statementMRS (data collection and draft-writing), MSF (conceptualization, data collection, and revisions), MBFG (data obtaining and revisions), JAB (conceptualization, data analysis and figures, and major editing).
FundingNone.
Data availabilityThe datasets are fully available in the Supporting Information files.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.