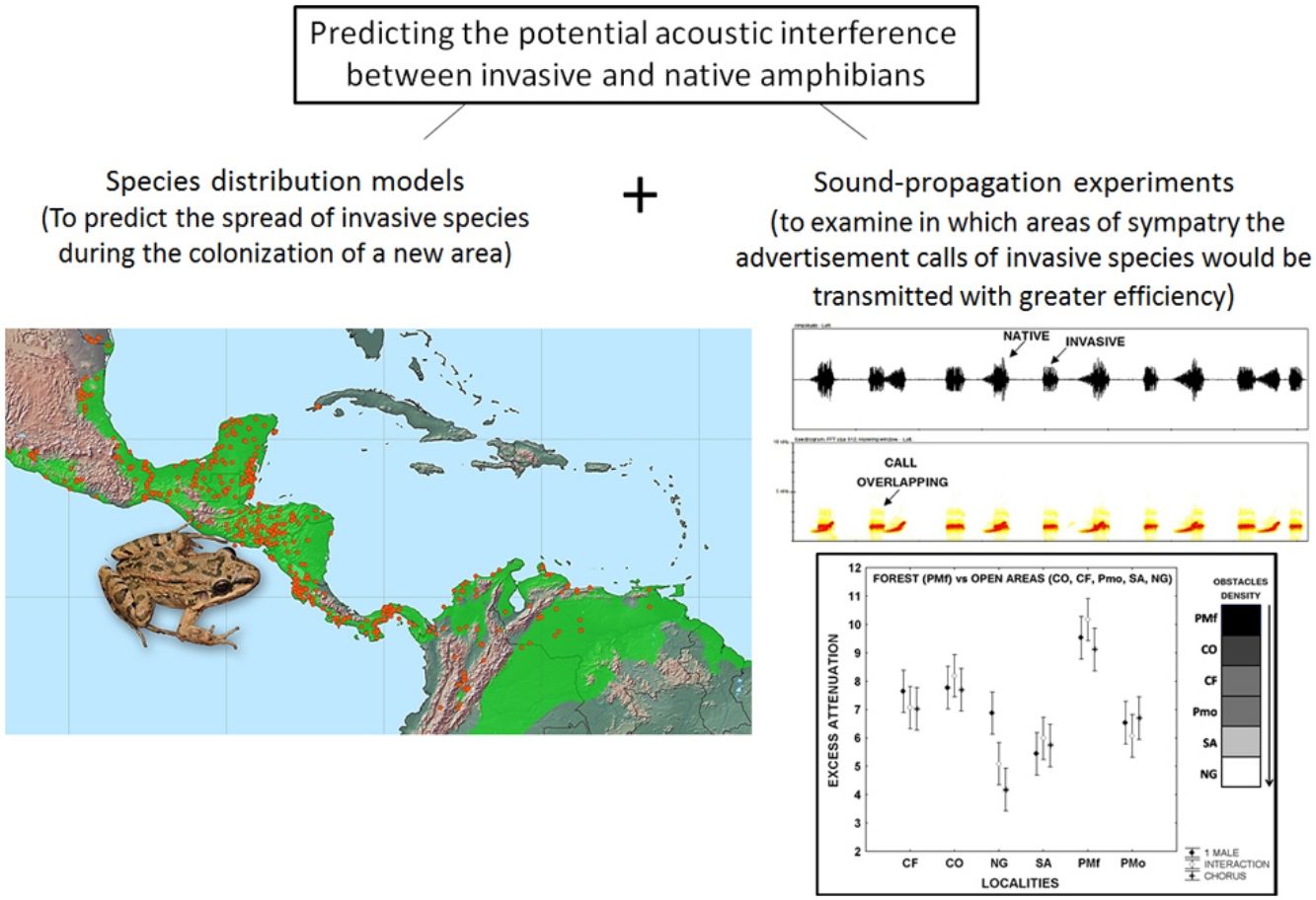
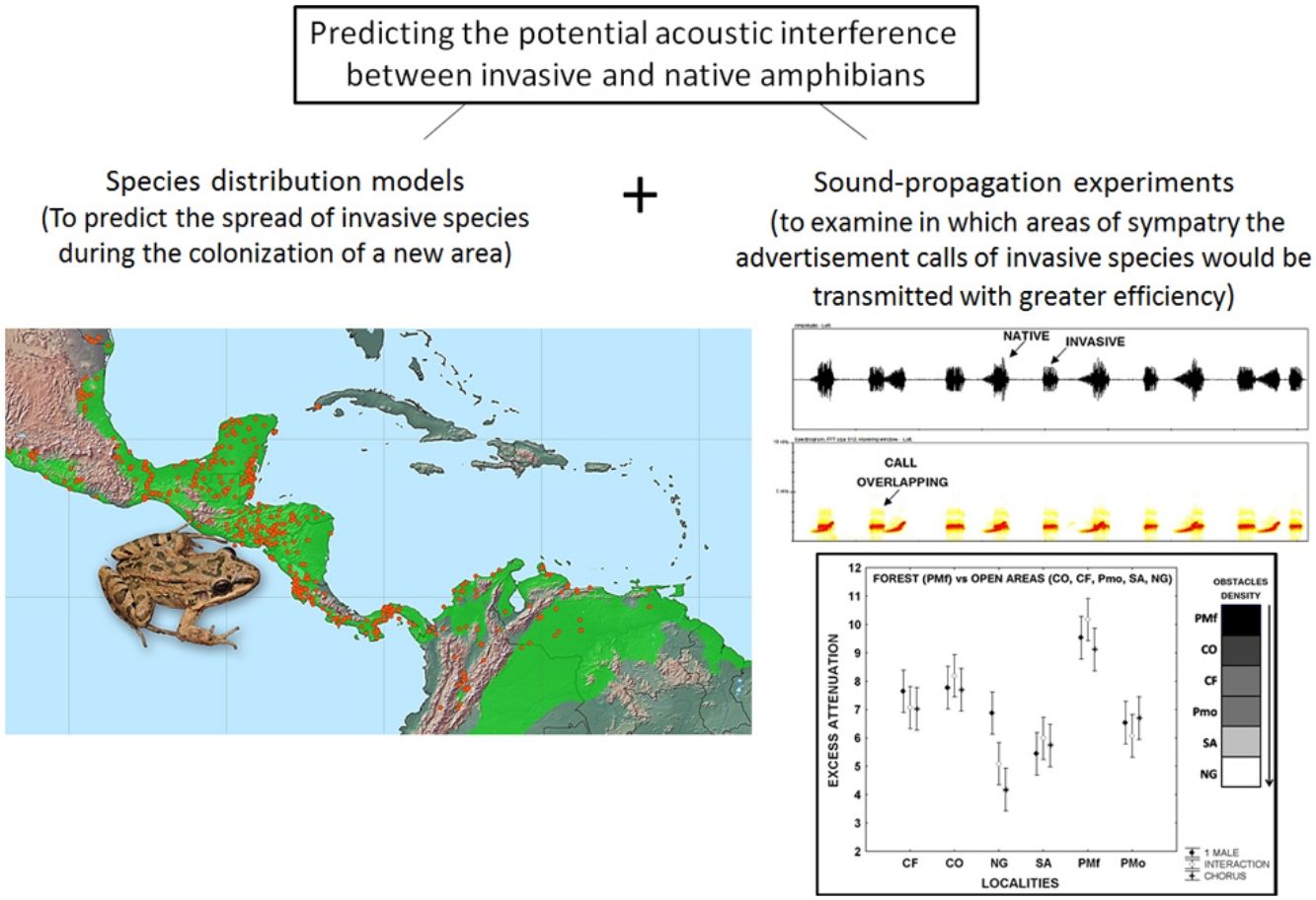
Leptodactylus fragilis is a recently introduced frog in Cuba, where it may impact local populations of amphibians in different ways. Here, we combined two methods to predict the invasion of the acoustic niche of Cuban amphibians by L. fragilis. We first use species distribution models to predict the spread and establishment of L. fragilis in Cuba. We then performed sound propagation experiments to evaluate the potential invasion of the acoustic niche in predicted suitable areas for the presence of L. fragilis. This species could have a successful establishment, spreading mainly in open areas, where its advertisement calls propagate efficiently, with low attenuation and discrete temporal-spectral degradation at short distances. The optimal transmission of acoustic signals in such areas might interfere with the acoustic communication of the endemic bufonid Peltophryne empusa, with potential negative effects on mate choice and breeding success. Predictions of habitat suitability, in combination with evidences of optimal transmission of advertisement calls of invasive amphibians, could be a valuable tool for rapid prediction of the potential impact of such species and the identification of prioritized areas to implement management strategies.
The global decline of amphibians appears to be a multifaceted phenomenon (Grant et al., 2020). In particular, biological invasions can cause severe impacts on native amphibian populations through competition, predation, hybridization, or alteration of the composition of native communities (Bucciarelli et al., 2014; Nunes et al., 2019). Invasive amphibian species can induce changes in the behavior of native species directly, through predation or interference competition, or indirectly, for instance through interfering with the intraspecific communication systems of native species. For example, following exposition to calls of invasive American bullfrog (Lithobates catesbeianus) native male white-banded tree frogs (Hypsiboas albomarginatus), immediately shifted calls to significantly higher frequencies, and continued to use higher frequencies while also decreasing signal duration during the post stimulus period (Both and Grant, 2012). On the other hand, Bleach et al. (2015) provided evidence that male Limnodynastes convexiusculus can adjust both, their calling rate and variance in inter-call interval in response to a variety of sounds, including the calls of the invasive Rhinella marina. In addition, male Li. convexiusculus called more intensively during the long silent gaps than during calling blocks. The acoustic niche of native species of amphibians might thus be severely altered through interference with vocalizations of invasive species. The acoustic niche comprises the microhabitat used for calling, the time during which calling activity takes place, and the advertisement call features (Sinsch et al., 2012). However, so far, studies are mainly limited to well-known invaders such as the American bullfrog Lithobates catesbeianus (Both and Grant, 2012), the Cane toad Rhinella marina (Medeiros et al., 2017), and the Cuban tree frog Osteopilus septentrionalis (Tennessen et al., 2016).
The White-lipped thin-toed frog, Leptodactylus fragilis (Brocchi, 1877), has a wide distribution, from southernmost Texas (USA), through Middle America to northern Colombia and northern Venezuela (de Sá et al., 2014). Adult White-lipped frogs can be encountered in cultivated fields, irrigated agricultural fields, irrigation ditches, low grasslands, and runoff areas (Garrett and Barker, 1987). Males of this nocturnal frog species have been found calling under clumps of grass, dirt clods, and from small depressions located just under the surface of the soil. Following the start of the rainy season, breeding begins when small pools fill with water, forming the right environment for nesting (Tipton et al., 2012). The female is attracted to the burrow where mating takes place and the foam nest is formed. Males call nearby incubating chambers that they defend against other males (Dixon and Heyer, 1968). The larval development is initiated in the burrow, after what larvae are released into the adjacent pond when the burrow becomes flooded following heavy rains (Dixon and Heyer, 1968).
Recently, Rodríguez-Cabrera et al. (2018) reported the presence of L. fragilis in two localities of western Cuba. Given the species’ natural history and its ability to exploit a variety of habitats (Heyer et al., 2006; de Sá et al., 2014), this invader could have a marked impact on Cuban amphibian populations. In particular, an overlap in the period of reproductive activity could affect the behavior, acoustic niche communication and spatial ecology of native species (Kraus, 2015).
In the present study, we explored the potential acoustic interference between L. fragilis and native amphibian species, as a first step toward predicting the potential consequences of the invasion process. To that end, we used both species distribution models and sound propagation experiments. We first predicted the establishment and spread of L. fragilis in Cuba. We then evaluated the potential invasion of the acoustic niche of native species in climatically suitable areas, analysing the efficiency of advertisement calls transmission, in terms of attenuation and degradation in sympatry.
MethodsSpecies distribution modelWe used ecological niche models (ENM) to identify regions in Cuba most likely to be colonized by L. fragilis. The ENM was first built using records of the native distribution of the species and bioclimatic variables, before being projected onto the Cuban archipelago to predict the potential distribution. The ENM was generated using Maximum entropy model (MaxEnt v.3.3.3, Phillips et al., 2006), that predicts habitat suitability as a function of environmental variables and species occurrence data. It relies on presence-background data (i.e., randomly selected absences from areas that have been accessible to the species; Phillips and Dudík, 2008), and is particularly suitable to explore the likely distributions of invasive species (Jiménez-Valverde et al., 2011; Cordier et al., 2020).
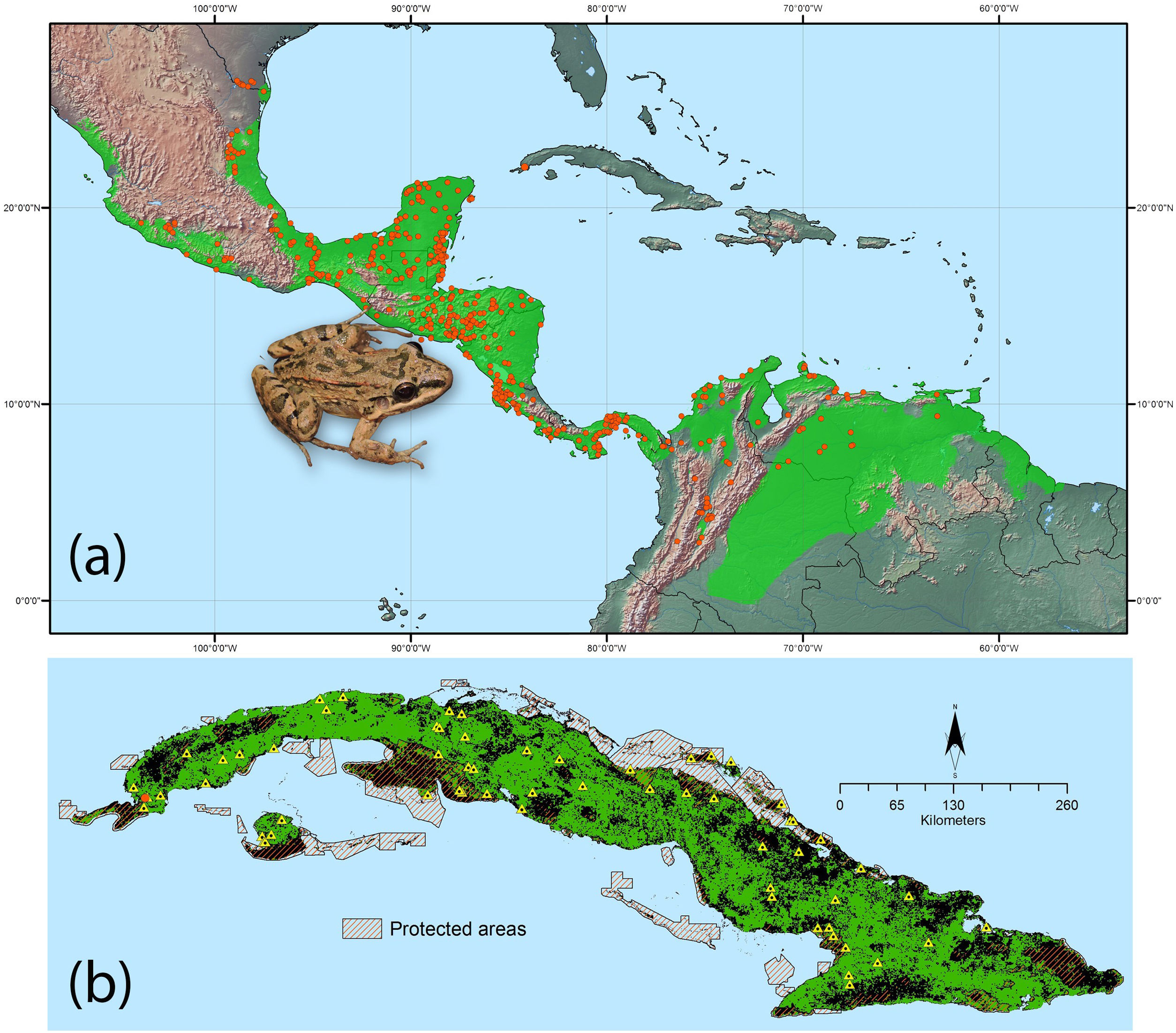
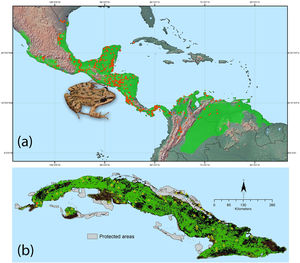
We obtained 551 georeferenced records describing the native distribution of L. fragilis in mainland America from Medina et al. (2020). To reduce clusters of localities that might create bias in environmental space, we used the R package spThin (Aiello-Lammens et al., 2015) to spatially filter occurrence records with a minimum distance of at least 5km. After filtering, 462 occurrence records remained for modeling distributions (Fig. 1a). To build ENM, we choose nine climatic variables: annual mean temperature (bio 1), mean diurnal range (bio 2), temperature seasonality (bio 4), maximum temperature of warmest month (bio 5), minimum temperature of coldest month (bio 6), annual precipitation (bio 12), precipitation seasonality (bio 15), precipitation of wettest quarter (bio 16), and precipitation of driest quarter (bio 17). Such bioclimatic variables were selected, as they are critical for amphibian survival and reproduction (Wells, 2007). Indeed, due to complex life cycle with aquatic and terrestrial life stages, shell-less eggs, permeable and exposed skin, and ectothermic condition, amphibians are very sensitive to changes in environmental quality (Hillman et al., 2008). All variables were extracted from Worldclim database 2.0, based upon weather conditions recorded between 1970 and 2000, with a grid cell resolution of 30 arc seconds (Fick and Hijmans, 2017; http://www.worldclim.org). Percent contribution of each variable was then calculated as detailed in Phillips et al. (2006). In order to reduce model complexity and multicollinearity, only six variables (bio 2, 4, 6, 12, 15 and 17) were retained for further analysis, based on their high contribution and low degree of correlation between themselves (|Pearson correlation|≤0.75). We restricted background sampling to a 100km radial buffer zone around each occurrence record. We considered this extent appropriate for background selection, as it does not include large regions that the species does not inhabit due to dispersal limitations and/or biotic interactions (Barve et al., 2011). We extracted 10 000 background random points within these buffers using ArcGIS v. 10.2 (ESRI, Redlands, California, USA).
Ecological niche model for Leptodactylus fragilis based on 462 occurrence records (orange points) from its native range, green shading indicates the suitable climatic area using “10th percentile training presence” threshold (a). Potential distribution of L. fragilis onto the Cuban archipelago (green area) based on ENM and suitable land cover, the orange spot indicates the known localities of L. fragilis; the records of presence of Peltophryne empusa (yellow triangles) from Alonso Bosch (2011) and Rivalta et al. (2014) (b). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
We tuned the value of the regularization multiplier and determined the optimal selection of feature classes using the R package ENMeval (Muscarella et al., 2014). Regularization values ranged from 0.5 to 3, in increments of 0.5. Five settings of feature classes were evaluated: linear (L), linear and quadratic (LQ), hinge (H), LQH, and LQH plus product (P). The complexity of each model was evaluated using Akaike Information Criterion corrected for small sample sizes (AICc) (Warren and Seifert, 2011). We also inspected the predictive power (omission rate E=10%) and the overfitting by calculating AUC difference, which is the difference between AUC calculated on training localities and AUC calculated on evaluation localities (Warren and Seifert, 2011). To evaluate the selected model, we used the area under the curve (AUC) of the partial ROC (receiver operating characteristic; pROC), which allows differential weighting of omission and commission errors, and more accurately assesses the quality of the model (Peterson et al., 2008). Using program NicheA (Qiao et al., 2016), pROC was used as a measure of model performance, with pROC ranging from 0 to 2, where 2 indicates a perfect prediction and values <1 indicate predictions no better than random (Peterson et al., 2008).
We used clamping which treats variables outside the training range as if they were at the limit of the training range (Elith et al., 2010). The similarity between calibration areas and Cuban archipelago (novel area) was assessed using Multivariate Environmental Similarity Surface (MESS) implemented within Maxent. This procedure allows the identification of areas with high extrapolation for which obtained results should be interpreted with caution (Elith et al., 2010). The final model was run in MaxEnt using 50 bootstrapping replicates and the combination of regularization multiplier and feature classes with lowest AICc using all thinning occurrence records; this model was projected onto the Cuban archipelago. To obtain presence – absence map from the logistic values of environmental suitability (continuous probability from 0 to 1), we used the 10th percentile threshold, which is poorly sensitive to extreme environmental values (Radosavljevic and Anderson, 2014), reduces commission errors, and produces more conservative distributional maps (Liu et al., 2005). Finally, we applied a habitat filtering approach, removing areas with unsuitable land cover types from the climatic niche model projected to Cuban archipelago; this procedure acts to reduce commission errors inherent to the ENM based exclusively on climatic data (Rondinini et al., 2006). After extracting cover types from GlobCover project v. 2.3 (E.S.A., 2010), we reclassified the map with all categories suitable for L. fragilis (e.g. croplands, grasslands and shrublands) being grouped in a single class.
Recordings and analyses of advertisement calls of L. fragilisRecordings were obtained from six individuals at Sandino (22.075837N, −84.219685W, 9.5masl), Pinar del Río province, using a digital audio field recorder (Sony PCM-M10) with a directional microphone (Senheiser MEE/K6). Advertisement calls were obtained at a 44,100Hz sampling frequency, with a 16 bit resolution. The analysis of calls was performed with the Raven 1.3 software (Bioacoustics Research Program, Cornell Laboratory of Ornithology, 2012), using Hanning window, FFT size 2048, and overlap of 95%. From the oscillograms, call duration, interval between calls (error 0.001s), and number of pulses in each call were recorded, while dominant frequency to the nearest 0.02kHz at the peak of maximum amplitude were obtained from the power spectrum.
In order to assess the possible overlap in mean values of temporal (call duration, pulses per call and pulse rate) and spectral (dominant frequency) features of advertisement calls between L. fragilis, and native species, we examined the acoustic features of the advertisement calls of more than 60 Cuban species of frogs and toads (Díaz and Cádiz, 2006, 2008; Alonso Bosch, 2011). Special attention was devoted to species potentially occurring in sympatry with the invasive species in open areas, particularly those who vocalize and breed in temporary and permanent water bodies (Alonso Bosch et al., 2007; Díaz and Cádiz, 2008).
Playback experiments: degradation and excess attenuation of the advertisement callsPre-recorded signals used as playbacks were broadcasted using a loudspeaker placed in the natural environment in order to measure propagation efficiency, similar to previous experiments with other anuran amphibians (Kuczynski et al., 2010; Llusia et al., 2013; Bleach et al., 2015; Penna and Moreno-Gómez, 2015; Schwartz et al., 2016). Propagation experiments were carried out during the wet season, between 18:00 and 20:00h. The audio file for the experiment was designed using the software Adobe Audition CC 6.0 (Compilation 732, 64 bits). The 3-min long playback (44,100Hz and 16 bit) included sequences of pure tones of 1kHz, and a series of three different stimuli: consecutive calls of one male, vocal interaction between two males, and a chorus of L. fragilis (Appendix S1 file). Sound pressure level (SPL) was standardized for all the stimuli at 0.5m from the source.
The playback stimuli were broadcast with a self-powered loudspeaker (Pignose No.7-100) connected to a laptop computer and placed at positions typically occupied by calling males on the ground. The broadcast signals were recorded with a directional microphone (Sennheiser MEE/K6) pointing at the source of the playback, placed successively at distances of 0,5; 1; 2; 4; 8m from the loudspeaker, and a digital audio field recorder (Sony PCM-M10). At the same positions, a sound level meter (RadioShack, Error=2dB) was placed to obtain dB values of the signal at each distance. Relative humidity and air temperature were measured at each distance using a digital thermo-hygrometer (Winflex, Error=±1°C; ±5%RH).
We selected six localities to perform sound propagation experiments, considering the potential distribution of L. fragilis, the description of its advertisement call, and the potential overlapping with the structure of advertisement calls of native syntopic species. In order to compare the excess attenuation and degradation of advertisement calls of L. fragilis, sound propagation experiments were conducted in forest (PMf) and open (PMo) habitats of the ancient Botanical Garden of Havana (23.1001N, −82.3985W, 32.3masl.). The first habitat is a perturbed mesophyllous semideciduous forest with herbaceous stratum bears grasses, ferns and abundant lianas, while the second is located in an open area on the border of the forest. Two other localities in the main island were included in the study: Sandino, Pinar del Río, province (22.075837N, −84.219685W, 9.5masl.) and near to Campo Florido town in La Habana province (23.128944N, −82.157216W, 31.1masl.). Sandino (SA), one of the two previously known localities of L. fragilis in Cuba, corresponds to the shore of a temporary small pond surrounded by grass pastures and bushes, while Campo Florido (CF) is a very irregular terrain near a temporary stream surrounded by grass pastures and several small bushes. Additionally, two open habitat localities were selected from Isla de la Juventud: surroundings of El Colony (21.633332N, −82.983118W, 6.7masl.) and outside of Nueva Gerona city (21.870908N, −82.79291W, 22.5masl.). The study area in El Colony (CO) is a temporal flooded depression with abundant thin grass forming like a mattress, while Nueva Gerona (NG) is a grass pasture area, with a sandy soil substrate.
In order to assess the possible degradation of the signals, sequences of advertisement calls of one male and the interaction between two males for all distances (0,5; 1; 2; 4; 8m) were analyzed. Call duration (CD) was measured at zero amplitude level on the oscillogram (error=0.1ms), while the number of pulses per call (NP) was counted. The dominant frequency (DF) was measured to the nearest 0.02kHz, at the peak of maximum amplitude in the power spectrum. All of these features were measured on the advertisement calls of one male for 10 alternate calls, starting on the second call of the sequence. For the sequence of the interaction between two males, the same procedure was followed, using the call of the closest male. Considering that signal attenuation is a major effect of sound transmission that may interrupt effective communication if signals are attenuated below auditory thresholds of recipients, attenuation effects were calculated with the equation: spherical transmission loss (dB)=20log[dB Sound Pressure Level (SPL) at far distance (m)/dB SPL at 0.5 (m)] (Llusia et al., 2013). Excess attenuation was calculated by subtracting values of spherical spreading from the actual transmission loss (differences between SPL at 0.5m from the loudspeaker and the corresponding higher distances) measured with the sound level meter for 1, 2, 4 and 8m relative to measurement at 0.5m.
We obtained values of intensities for all studied localities and three broadcasted stimuli only when testing at a distance of 0.5–1m. At 0.5–2m values were obtained only for the three open localities for three stimuli, whereas the sensitivity of the equipment was too low to perform experiments at distances of 0.5–4m and 0.5–8m. Increasing the sensitivity of the sound level meter to its maximum value at the farthest distances from the loudspeaker had no effect on detectability.
Statistical procedureWe calculated mean and standard deviation for each parameter included in our analyses. For the analysis of variation of three acoustic variables along five distances among localities, Inverse Gaussian response distribution was selected by the lowest values of Akaike Information Criterion (AIC) after SEVERITY procedure, and reciprocal link function, while the analysis of excess attenuation along five distances, Gamma distribution and log link function were selected. We used a two-way Analysis of Variance, and Type III sums of squares, in a generalized linear mixed model with PROC GLIMMIX (Littell et al., 2006). We considered distance, locality and their interaction as fixed-effect factors. If there was an overall significant effect, we conducted Tukey–Kramer adjusted multiple comparison tests between all levels. For all analyses, the best-fit model was selected using AICC (Burnham and Anderson, 1998). All statistical analyses were conducted using SAS software version 9.3 (SAS Institute Inc., Cary, NC, USA), with significance level at α=0.05.
ResultsSpecies distribution modelThe ecological niche model of L. fragilis (Fig. 1a) with lowest values of AICc (delta AICc=0) resulted from regularization multiplier of 2.5 and LQHP feature class. The mean AUCdiff (0.025±0.0006 SD) and mean 10% training omission rate (0.12±0.0003 SD) were indicative of good model performance and low overfitting. pROC value was equal to 1.49 and significantly different from 1 (P<0.001), indicating significant predictive ability of model. The analyses of variable contributions revealed that minimum temperature of coldest month, temperature seasonality and mean diurnal range had the highest explanatory power (Appendix S2 File). Geographic projections of ENM showed climatic suitability for L. fragilis throughout the Cuban archipelago (Fig. 1b). Clamping and multivariate environmental similarity surfaces (MESS) showed that no areas had variables outside of the training data range. The ENM filtered with suitable land cover indicated that 66,930km2 are highly susceptible to invasion by L. fragilis, of which 5680km2 are included within the Cuban system of protected areas (Fig. 1b).
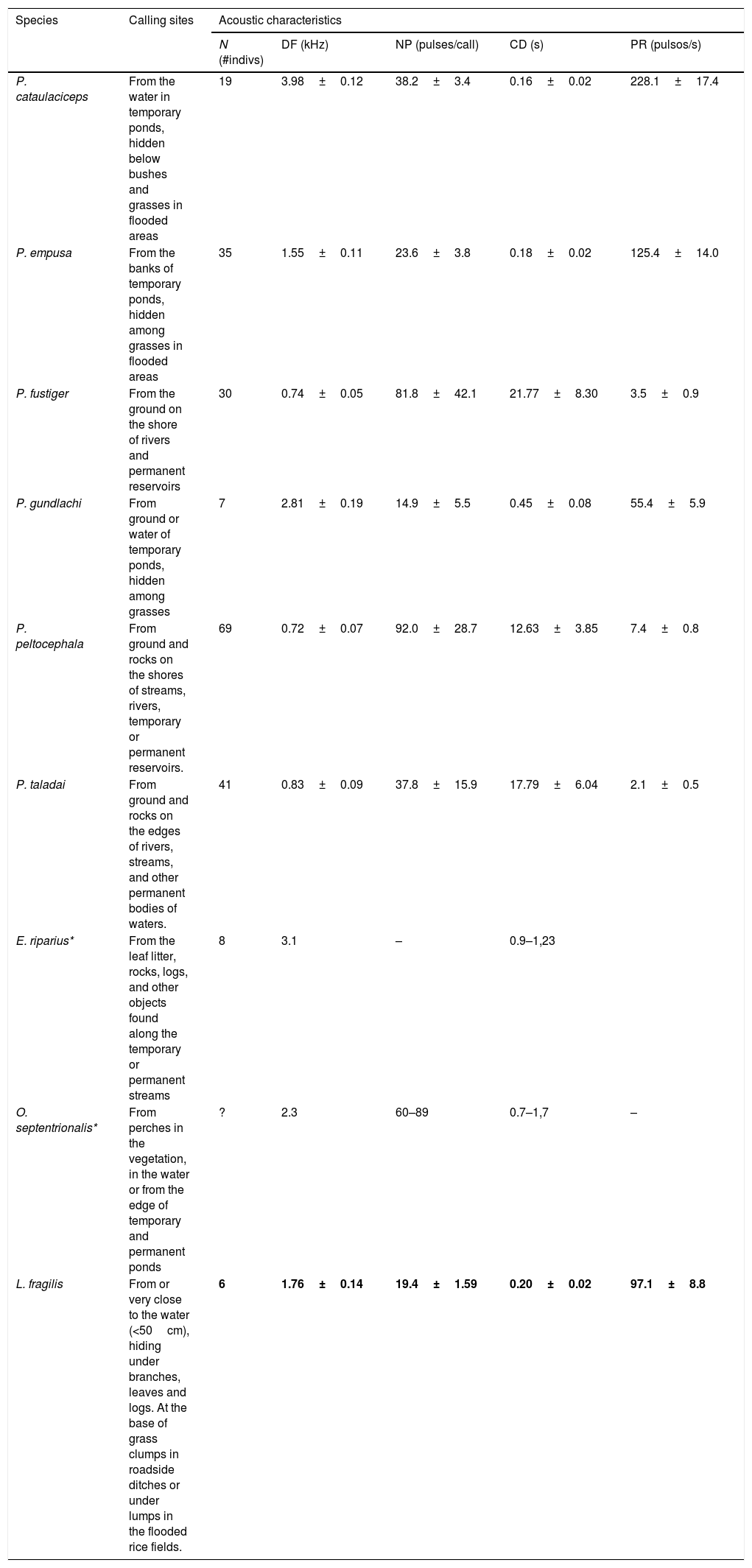
Advertisement call of L. fragilisMales of L. fragilis emit an amplitude-modulated advertisement call with harmonic structure. The advertisement call consists of a single pulsed note with 19.4±1.5 pulses (16–22), and two or three indistinct pulses added at the end. Its dominant frequency ranged between 1.38–2.07kHz (Mean±SD, 1.76±0.14kHz). Acoustic features of advertisement calls of L. fragilis, and those of eight native species whose vocal activity and mating take place more frequently in lentic water bodies in open areas, are provided in Table 1. The advertisement calls of Peltophryne empusa appear to be most similar in temporal and spectral features to L. fragilis relatively to the other species of toads we analyzed (Table 1). According to the previously obtained ecological niche model of L. fragilis in Cuba, this introduced species could be widely sympatric with the endemic P. empusa (Fig. 1b). In the hypothetical case where both species coexist in the same locality, share the microhabitat for vocalizing, and their calling activities coincide in time and space, the physical features of their advertisement calls could largely overlap (Table 1).
Calling sites and acoustics characteristics of the advertisement calls of native Cuban anurans (Peltophryne, Eleutherodactylus and Osteopilus) that frequently breed near or inside to of water bodies in open areas of the Cuban archipelago. For each variables (DF; dominant frequency; NP: number of pulses; CD: call duration; PR; pulse rate), Mean and Standard deviation (X±SD) are shown when it has been informed. Acoustic features of the advertisement calls of Leptodactylus fragilis obtained in this study are included in bold for comparative purpose. Sources: Alonso Bosch et al. (2007), Díaz and Cádiz (2008)*, Alonso Bosch (2011) and Rodríguez-Cabrera et al. (2018).
| Species | Calling sites | Acoustic characteristics | ||||
|---|---|---|---|---|---|---|
| N (#indivs) | DF (kHz) | NP (pulses/call) | CD (s) | PR (pulsos/s) | ||
| P. cataulaciceps | From the water in temporary ponds, hidden below bushes and grasses in flooded areas | 19 | 3.98±0.12 | 38.2±3.4 | 0.16±0.02 | 228.1±17.4 |
| P. empusa | From the banks of temporary ponds, hidden among grasses in flooded areas | 35 | 1.55±0.11 | 23.6±3.8 | 0.18±0.02 | 125.4±14.0 |
| P. fustiger | From the ground on the shore of rivers and permanent reservoirs | 30 | 0.74±0.05 | 81.8±42.1 | 21.77±8.30 | 3.5±0.9 |
| P. gundlachi | From ground or water of temporary ponds, hidden among grasses | 7 | 2.81±0.19 | 14.9±5.5 | 0.45±0.08 | 55.4±5.9 |
| P. peltocephala | From ground and rocks on the shores of streams, rivers, temporary or permanent reservoirs. | 69 | 0.72±0.07 | 92.0±28.7 | 12.63±3.85 | 7.4±0.8 |
| P. taladai | From ground and rocks on the edges of rivers, streams, and other permanent bodies of waters. | 41 | 0.83±0.09 | 37.8±15.9 | 17.79±6.04 | 2.1±0.5 |
| E. riparius* | From the leaf litter, rocks, logs, and other objects found along the temporary or permanent streams | 8 | 3.1 | – | 0.9–1,23 | |
| O. septentrionalis* | From perches in the vegetation, in the water or from the edge of temporary and permanent ponds | ? | 2.3 | 60–89 | 0.7–1,7 | – |
| L. fragilis | From or very close to the water (<50cm), hiding under branches, leaves and logs. At the base of grass clumps in roadside ditches or under lumps in the flooded rice fields. | 6 | 1.76±0.14 | 19.4±1.59 | 0.20±0.02 | 97.1±8.8 |
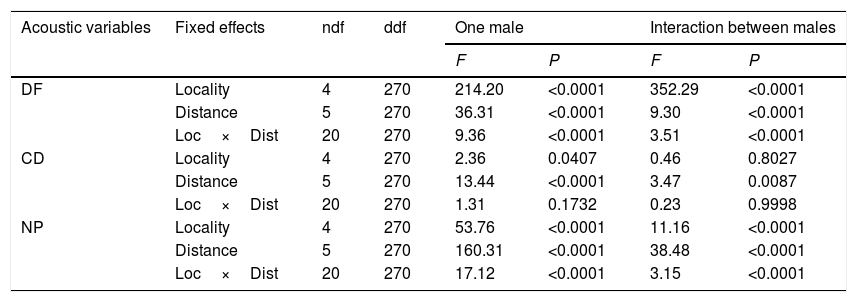
Although we did not observe any severe degradation in the signals of L. fragilis, significant effects of distance and locality (P<0.001) were observed (Table 2, Appendix S3 File). Call duration was the most stable acoustic feature, when considering either the signal of one single male or the interaction between two males (Table 2, Appendices S4 and S5). Locality had no significant influence on the three acoustic features of one single male call (Table 2, Appendix S4). In terms of call duration of one single male, Nueva Gerona (NG) was the most different locality (with less variation in relation to the stimuli), whereas this variable for the interaction between males, was very similar among localities (Appendix S5). The number of pulses in both cases (one single male and interaction) followed the same pattern: all localities were significantly different from Nueva Gerona and Sandino (SA), whereas the latter two did not differ between themselves. Finally, the comparative analysis of the dominant frequency among localities did not reveal a clear pattern. However, Campo Florido (CF) and both areas in the ancient Botanical Garden of Havana [PMo (Open) and PMf (Forest)] appeared to be the most distinct localities, with the highest variation for both the advertisement calls of one single male and the interaction between males (Appendices S4 and S5).
Generalized linear mixed models results of sound propagation experiments of advertisement calls of one male, and interaction between two males of Leptodactylus fragilis at five distances from the loudspeaker in six known localities of Peltophryne empusa distribution.
| Acoustic variables | Fixed effects | ndf | ddf | One male | Interaction between males | ||
|---|---|---|---|---|---|---|---|
| F | P | F | P | ||||
| DF | Locality | 4 | 270 | 214.20 | <0.0001 | 352.29 | <0.0001 |
| Distance | 5 | 270 | 36.31 | <0.0001 | 9.30 | <0.0001 | |
| Loc×Dist | 20 | 270 | 9.36 | <0.0001 | 3.51 | <0.0001 | |
| CD | Locality | 4 | 270 | 2.36 | 0.0407 | 0.46 | 0.8027 |
| Distance | 5 | 270 | 13.44 | <0.0001 | 3.47 | 0.0087 | |
| Loc×Dist | 20 | 270 | 1.31 | 0.1732 | 0.23 | 0.9998 | |
| NP | Locality | 4 | 270 | 53.76 | <0.0001 | 11.16 | <0.0001 |
| Distance | 5 | 270 | 160.31 | <0.0001 | 38.48 | <0.0001 | |
| Loc×Dist | 20 | 270 | 17.12 | <0.0001 | 3.15 | <0.0001 | |
The comparative analysis of L. fragilis signal degradation along five distances to the loudspeaker showed significant differences for the three studied acoustic variables, for both the advertisement calls of one single male and the interaction between males (Table 2). Noticeable variations were detected in terms of call duration and number of pulses when examining both stimuli (one male call and interaction between males) at the farthest distances (Appendices S4 and S5).
There was a significant interaction between distance to the loudspeaker and locality (Loc×Dist) on signal degradation (Table 2, Appendices S3–S5). The most relevant pattern was observed for the dominant frequency, with more structurally complex localities (higher number of obstacles and denser vegetation) being significantly different from more open ones at all distances (0.5, 1, 2, 4, and 8m to the loudspeaker). A similar pattern was detected both in the analysis of the signal degradation of the one single male call and in the interaction between males (Appendix S3). Differences in dominant frequency among open localities appeared only at the farthest distances. No particular pattern was observed for the remaining acoustic variables analyzed (Appendices S3–S5). We also checked for the effect of the interaction between distance and locality, when establishing comparisons between localities, considering different distances (Appendix S3).
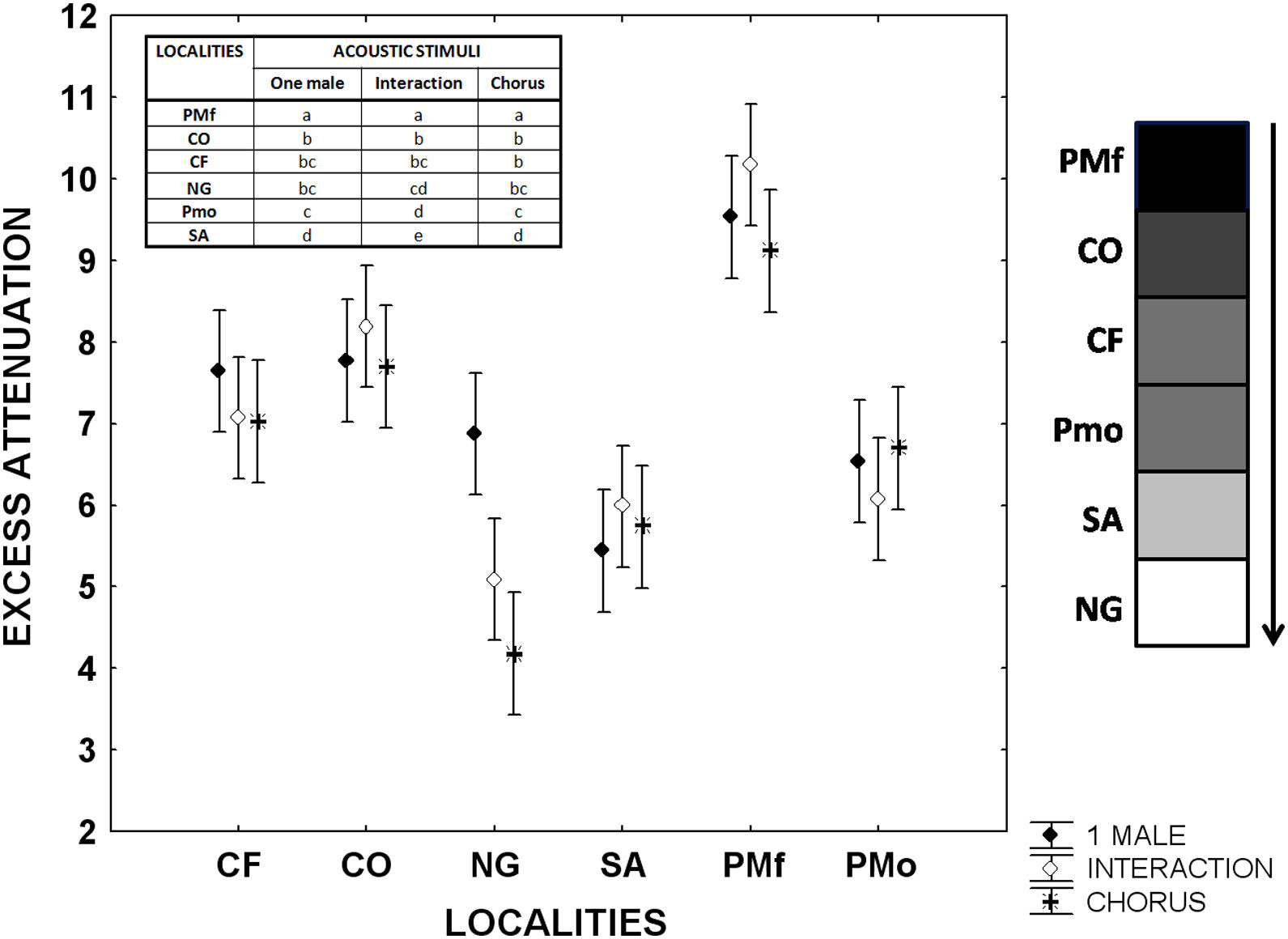
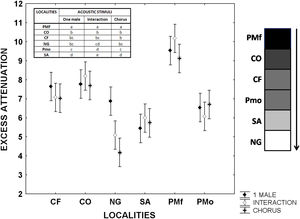
Advertisement calls of L. fragilis in open and forest areas: excess attenuationMeasurements of playback experiments between 0.5 and 1m from the loudspeaker in the forest of the ancient Botanical Garden of Havana (PMf) showed increased attenuation (P<0.001) of advertisement calls of one single male, interaction between two males, and chorus, compared to open areas (Fig. 2, Appendix S6). The excess of attenuation was very similar between Colony (CO), Campo Florido (CF), the open area in the ancient Botanical Garden of Havana (PMo) and Nueva Gerona (NG) at the first evaluated distance (Fig. 2, Appendix S6). However, the excess of attenuation in these open areas was also significantly different in relationship to the obtained values in Sandino (SA) at the same distance from the loudspeaker. This locality showed the lowest excess of attenuation for one single male call when performing tests at the 0.5–1m distance, while the excess of attenuation for both the interaction and chorus was minimal in Nueva Gerona (Fig. 2, Appendix S6). The excess of attenuation at the 0.5–2m distance differed among the open localities where the three stimuli were perceived by the instrument (CF, NG, SA), with Nueva Gerona showing the lowest values of attenuation for the three types of stimuli (Appendix S6).
Excess attenuation of sequences of the advertisement calls of one single male, an interaction between two males, and a chorus of Leptodactylus fragilis between 0.5 and 1.0m from the loudspeaker. Filled rhombus: advertisement call of one single male; Open rhombus: interaction between two males; Asterisks: chorus. CF: Campo Florido, CO: Colony, NG: outside Nueva Gerona, SA: Sandino, PMf: Forest in Parque Metropolitano of Havana city, Pmo: Open area in Parque Metropolitano of Havana city. For each locality mean and 95% confidence intervals are shown. Gray scale bar represents schematically the density of vegetation and obstacles for each locality. The results of the post hoc Tukey-Krammer test are also showed in the inserted table.
Our results strongly suggest an expansion of the invasive L. fragilis in Cuba in the next decades, particularly in open habitats where its advertisement call can propagate without much degradation or attenuation. Based on the degree of overlap in call characteristics with native species, we further predict that the native P. empusa might be particularly affected by the demographic expansion of L. fragilis.
According to our species distribution model, the bioclimatic conditions present in Cuba are suitable for the spreading of L. fragilis along the lowland areas of the archipelago. This pattern corresponds with the native distribution of the species in Central America, where it occurs mostly in low grassland, irrigated fields and roadside ditches (Heyer et al., 2006). It also agrees with the known distribution in Cuba, thus supporting the hypothesis of climatic niche conservatism for this invasive species, spreading primarily in regions that are climatically similar to their native range (Wiens et al., 2010; Peterson et al., 2011).
In addition, our results indicate that the expansion of the invasive amphibian should occur mainly in open habitats. Indeed, we observed a higher attenuation of the three sequences of advertisement calls of L. fragilis, consistent with previous reports of a more pronounced attenuation and degradation in densely vegetated habitats (Gerhardt and Huber, 2002). This is explained by the fact that the interaction of acoustic signals with environmental objects can cause scattering and reflections, changing some spectral and temporal patterns of the call, that are thus being perceived in a more diffuse way by receivers (Bradbury and Vehrencamp, 2011). Such interactions can also decrease the amplitude of acoustic signals, and increase the attenuation expected by spherical spreading (Gerhardt and Huber, 2002). Although we observed discrete variations in substrates and vegetation among studied localities, with the exception of the forest area where the signal appears to suffer more severe degradation and attenuation, remaining habitats and weather conditions are suitable for the advertisement call transmission and, consequently, for the establishment of L. fragilis. Better call transmission efficiency in open areas should result in a more effective communication between conspecifics and a higher probability of reproductive success. In addition, the weak evidence for acoustic degradation suggests that the intraspecific communication of L. fragilis should not be much affected in open habitats, as this species usually vocalizes in dense chorus, with little distance between calling males. For instance, Rodríguez-Cabrera et al. (2018) observed several males (>40 individuals) calling in dense choruses, spaced about 1m apart of each other. Our observations in Sandino suggest that the density of calling males varies along the year, although during the rainy season we could observe individuals calling at less than 50cm from each other.
Previously, Rodríguez-Cabrera et al. (2018) observed that L. fragilis was found in syntopy with other native riparian or aquatic-breeding anurans of three different families (Eleutherodactylidae, Hylidae, Bufonidae), including the Cuban small-eared toad. All these species stopped their vocalizations when the chorus of L. fragilis became more intense. Our results suggest that L. fragilis could largely interfere with P. empusa, which is widely distributed in lowlands and savannahs throughout most of the Isle of Cuba, Isla de la Juventud, and in some keys from the Sabana-Camaguey archipelago (Alonso Bosch, 2011). This toad inhabits savannas, grasslands, open areas of crops, swampy regions and other flat areas that are flooded with the rains (Díaz and Cádiz, 2008). Adult P. empusa are primarily nocturnal and seek refuge in burrows by day and during the dry season, whereas juveniles tend to be more active during the day. Typical of an explosive breeding species, individuals remain in burrows below ground outside of the breeding season (Díaz and Cádiz, 2008). During the reproductive season, similar to L. fragilis, male P. empusa vocalize at night in dense chorus in open areas, on the banks of temporary ponds, hidden among grasses, flooded ditches, and flooded road ruts following very heavy rains (Alonso Bosch et al., 2007). They can also call from cylindrical cavities in the mud, with a 10–15cm water depth (Garrido et al., 1986). Eggs are laid in still water from temporal ponds formed by rain in open areas of savannas and pastures. Hatching occurs between 30 and 37h and the larvae complete their metamorphosis in 13–18 days (Díaz and Cádiz, 2008).
L. fragilis could interfere directly with the native P. empusa, through disrupting conspecific communication and affecting mate selection and reproductive success (Both and Grant, 2012; Bleach et al., 2015; Tennessen et al., 2016; Medeiros et al., 2017). Although we acknowledge that the advertisement calls of L. fragilis and P. empusa are in different acoustics guilds (Emmrich et al., 2020), our results show that acoustic features of the advertisement calls of L. fragilis exhibit some similarities to P. empusa calls. Both species use similar spectral band and their signals are comparable in terms of call duration. In anurans aggregations, when the availability of breeding sites is spatially and temporally limited, strong competitive pressure from conspecific and heterospecific individuals can take place. Such competitive interactions are usually associated with access to food, calling space, and the frequency of calls (Mullet et al., 2017). In case of strong overlapping (either spectral or temporal) between species, individuals might be forced to increase energy expenditure, increase signal duration, or switch the vocalization frequency, with possible negative fitness consequences (Wong et al., 2009; Luther and Gentry, 2013; Bleach et al., 2015). We conclude that there is a high probability of invasion of the acoustic niche of P. empusa by L. fragilis, considering that the transmission of advertisement calls of the introduced species tends to be optimal in open areas inhabited by the native species. The potential spreading of L. fragilis in Cuba, with the consequent possible interference of acoustic niche, thus represents a threat for the reproductive success and population stability of P. empusa.
Rapid recognition of invasive species is crucial in order to evaluate and manage potential impacts (Toledo and Measey, 2018). Although our main predictions remain to be checked in the future through regular close monitoring of L. fragilis in Cuba, our study shows that the combination of species distribution models and sound propagation experiments could be a promising tool to predict both the dynamics and impact of invasive amphibian species. Our results could also be useful in the future design of strategies for the control and management of L. fragilis species by means of manipulations of acoustic signals. In particular, broadcasting manipulated advertisement calls might be used as lures to attract males and females in areas offering suitable environmental conditions for its establishment and reproduction (Muller & Schwarzkopf, 2017; Groffen et al., 2019; Muller et al., 2020).
Declaration of Competing InterestThe authors report no declarations of interest.
We thank Caribaea Initiative (grant CI-201903R) for financial support. This study is part of the Master project in Behavioural Ecology and Wildlife Management of SLCD, who is thankful to the Université de Bourgogne–Franche Comté and the Programme Investissement d’Avenir for acceptance and financial support. L. Yusnaviel Garcia provided valuable assistance during the recording sessions and sound propagation experiments. Juan L. Leal, Mónica Herrera, Ana K. Durán, and Laura Pérez helped us during the fieldwork too. Many people from the studied localities offered their logistic facilities and hospitality. We are very thankful to Dr. Rafael Márquez for the review of an earliest version of the manuscript and his useful comments. The manuscript also benefitted from helpful comments from three anonymous reviewers.