Other effective area-based conservation measures (OECMs) have recently been implemented in countries such as Colombia and, together with protected areas (PA), are crucial biodiversity conservation strategies. Assessing the contribution of different area-based conservation frameworks (i.e., PA and OECMs) involves evaluating the representation degree of species' geographic ranges, representation targets achievement (i.e., Gap analysis), priority areas for conservation, and their relationship with the remaining habitat. Snakes regulate prey populations, interfere with the behavior and diet of other species, can be bioindicators, and facilitate the transfer of energy and biomass between environments, making them a conservation priority. Currently, Colombia hosts > 300 snake species from nine families. Here, we explored the snake diversity pattern in Colombia and its relationship with remaining habitat. We also evaluated the degree of representation within PA and OECMs of species geographic distributions, species richness, and priority areas for conservation. Areas with the highest snake richness are in the Andean, Pacific, and Amazon regions; however, these are predominantly outside PA and OECMs. Representativeness of species ranges and representation targets within PA increased with the OECMs. The Caribbean and Andean regions have areas with the lowest remaining habitat. Our findings highlight that the OECMs contribute to the conservation of snakes in Colombia and complement PA. The Pacific, Orinoco, Amazon, and the northern Caribbean presented the highest concentration of priority areas for conservation and given the presence of indigenous people groups and large remaining habitat, these regions are most promising for creating new OECMs.
Globally, biodiversity has been affected by the increasing expansion of anthropized areas; for example, between 1960 and 2019, 32% of the global area showed changes in land use, mainly due to the expansion of agricultural land (Winkler et al., 2021). In South America, human activities affected 40% of land cover in 2018 (Zalles et al., 2021). Specifically, in Colombia, livestock, agriculture, mining, oil extraction, and illicit crop development are the major causes of natural cover loss (Andrade and Castro, 2012; Palacio et al., 2001). Paradoxically, natural cover loss worsened after the 2016 peace agreement, particularly in areas previously occupied by FARC-EP guerrillas (Fuerzas Armadas Revolucionarias de Colombia Ejército del Pueblo), which have been mainly used for illicit crops development (Pirela-Ríos et al., 2023). Understanding the processes that drive biodiversity loss could help manage areas destined for production and biodiversity conservation (Margules and Pressey, 2000).
Area-based conservation frameworks (e.g., protected areas –PA– and other effective area-based conservation measures –OECMs–) are a globally applied approaches that define geographic areas for in situ conservation of biodiversity (Salafsky et al., 2024). PA are geographic areas dedicated to the long-term conservation of biodiversity, ecosystem services, and cultural values (CBD, 2018). PA contribute to the sustainment of ecosystem services (Figgis et al., 2015), are a source of resources for human communities (Velazco et al., 2022a), and enable the survival of biodiversity in the face of global changes (Lehikoinen et al., 2021; Thomas et al., 2012). Thus, PA has become one of the main tools to address biodiversity loss (Margules and Pressey, 2000; UNEP-WCMC, IUCN, 2021).
Alongside PA, OECMs have been implemented internationally. OECMs are areas not designated as PA but managed in such a way that they conserve biodiversity; ecosystem functions and services; and cultural, spiritual, and socioeconomic values (CBD, 2018). A fundamental distinction between PA and OECMs is that PA are primarily designed to conserve biodiversity, whereas OECMs can be managed for a range of objectives, and conservation may or may not be the primary objective (IUCN-WCPA Task Force on OECMs, 2019). OECMs were proposed in the Strategic Plan for Biodiversity 2011–2020 and included in the Aichi Targets (CBD, 2010), and the Convention on Biological Diversity (CBD) ratified that target 3 can be achieved through PA and OECMs (CBD, 2022). Recently, it has been found that OECMs provide additional coverage and connectivity in different ecoregions, key biodiversity areas, and countries, which could significantly contribute to the achievement of conservation targets (Jonas et al., 2024).
Colombia’s National System of Protected Areas (SINAP) divides PA into public areas (e.g., national parks or protective forest reserves) and private areas (e.g., civil society reserve areas; Munévar and Ramírez, 2021). After COP 14, Colombia adopted the OECMs, allowing the development of a methodological route for identifying and reporting OECMs to the World Conservation Monitoring Center. Thus, Colombia has become an international reference and the first country in Latin America and the Caribbean to identify, nominate, and report the OECMs (Santamaría, et al., 2021). Therefore, the protected continental area in Colombia represents 31%, reaching CBD target 3 (30 × 30), which states that by 2030, at least 30% of the terrestrial, inland water, marine, and coastal areas should be protected (Dinerstein et al., 2019). Despite this progress, there has been evidence of a reduction in natural cover within PA and their surrounding regions due to deforestation, fires, and land-use changes in Colombia (Murillo-Sandoval et al., 2018).
Globally, PA networks exhibit biases in effectively representing biodiversity and connectivity between them (Saura et al., 2017; Sayre et al., 2020). This is because PA have often been established in isolated, sparsely populated places or are unsuitable for cultivation (Baldi et al., 2017). PA efficiency can be evaluated through the representativity of species ranges (or other biodiversity attributes) within PA using spatial conservation prioritization or Gap analysis (Kukkala and Moilanen, 2013; Rodrigues et al., 2004). Several studies have shown that PA biases reduce their efficiency (e.g., Gomes et al., 2024; Lourenço-de-Moraes et al., 2019; Oliveira-Dalland et al., 2022). However, OECMs could suffer from the same biases, and because they are relatively new area-based conservation frameworks, research on their conservation contributions and how they complement PA are still scarce (Cook, 2024).
Habitat loss and degradation are factors that decrease reptile populations (Gibbons et al., 2000); nevertheless, studies on the loss of reptile diversity in response to land-use change remain rare (Andrade Correa, 2011). Globally, integrated studies on the conservation status of reptiles are scarce compared to other vertebrates, hindering effective conservation strategies (Cox et al., 2022). Snakes play different roles in terrestrial and aquatic ecosystems, and because of their position across various trophic levels, they affect the behavior and diet of other species (Adams et al., 2024; Willson and Winne, 2016). Because of the predatory behavior of snakes, they can regulate prey animal populations (e.g., rodents, insects, amphibians; Lynch, 2012; Title et al., 2024), control pest populations (Shine et al., 2024), and may even act as a secondary dispersal (Reiserer et al., 2018). In addition, they facilitate the transfer of energy and biomass between aquatic and terrestrial environments (Willson and Winne, 2016). A decrease in snake populations could lead to destabilization of ecosystem processes (Adams et al., 2024). Further snakes' ecosystem roles, they can serve in ecological risk assessments (Campbell and Campbell, 2001; Weir et al., 2010), bioindicators (Ugochukwu et al., 2024), and their poison used to derive new drugs (Oliveira et al., 2022). Therefore, snakes are an important group for studying their diversity patterns, their relationships with land use, and quantifying the contribution of different area-based conservation frameworks (i.e., PA and OECMs) to their protection.
Habitat loss and death by farmers are the main factors affecting snake survival in Colombia and, to a lesser extent, road mortality and wildlife trafficking (Lynch, 2012). In Colombia, 9% of reptiles are under some degree of threat, and 20% are Data Deficient; particularly for snakes, ten species are under some threat category (Galvis et al., 2016). Colombia has nine families within the suborder Serpentes, of which Colubridae is the most diverse and abundant (Lynch et al., 2014). Snakes in this country are in most of the territory and distributed in an altitudinal range from 0 to 2600 m a.s.l. (Lynch, 2012). In this study, we applied species distribution modeling and spatial prioritization techniques to (i) assess the degree of snake representativeness within the PA and OECMs networks, (ii) determine the proportion of remaining snake habitat and (iii) evaluate the degree of representativeness within PA and OECMs of snake geographic distributions, species richness, and priority areas for conservation.
MethodsStudy areaColombia is crossed by the Andes mountain range, which branches into three mountain ranges, giving it a high orographic complexity (Rangel, 2010). The Baudó system, Sierra Nevada de Santa Marta, and Macarena also stand out, complemented by valleys and mountains that confer a variety of meso- and microclimates (IDEAM-UNAL, 2018). Seventy percent of Colombia has a mean temperature of 24 °C (IDEAM-UNAL, 2018). The rainiest areas (Pacific region, Amazonian foothills, and plains) can have rainfall >4000 m/year, whereas the driest regions (Guajira Peninsula, some regions of the inter-Andean valleys) have rainfall of 500 m/year (IDEAM-UNAL, 2018). Six natural regions are recognized in Colombia: the Andean, Pacific, Amazon, Orinoco, Caribbean, and Insular regions (Fig. S1; Rangel, 2010).
PA and OECMs databaseProtected areas in Colombia began in 1938, consolidated with the signing of the CBD in 1992, ratified in Law 165 of 1994 (Lenis, 2014), and in 1993 the SINAP - National System of Protected Areas was proposed (Lenis, 2014). In 2019 in Colombia, OECMs were identified, strengthened, and reported at the international level (Santamaría et al., 2021). By 2023, Colombia had 1652 PA and 48 OECMs, encompassing 187,817 and 128,480 km2, respectively (SINAP, 2023; UNEP-WCMC, 2024; Table S1). The Protected Planet database (https://www.protectedplanet.net) was used as source of the PA and OECMs.
Remaining habitatWe calculated a remaining habitat map to evaluate the extent to which each species range was affected by habitat loss in 2022 (Appendix S1 in SM). Values of remaining habitat map closer to 1 indicate landscapes with a higher proportion of habitat (Table S2). We graphically explored the relationship between potential snake species richness (i.e., based on stacked species distribution models of semi-binary models; see details below) and remaining habitat both in geographic space and at the cell level (Velazco et al., 2023).
Compilation, integration, and cleaning of snake recordsWe obtained a list of 334 snake species native to Colombia from the Reptile DataBase portal (Uetz et al., 2023). We then reviewed the literature and other data sources for Colombia (BioModelos, 2023; SIB Col, 2023) to eliminate species with insufficient information on their occurrence. Species records were compiled from various open-access databases (Table S3). To better represent the environmental requirements of the species, models were constructed with records from throughout species' natural range (i.e., inside and outside Colombia). Taxonomic errors, geographic inaccuracies, and biases in record data lead to decreased performance of species distribution models (SDMs) and alterations in diversity patterns (Baker et al., 2022; Maldonado et al., 2015). We used the R bdc package (Ribeiro et al., 2022) to integrate record databases and perform spatial and temporal corrections (Appendix S2). Final database comprised 56,830 records and 309 species. Furthermore, for those species with no occurrences, <3 occurrences, or low model performance (i.e., Sorensen values < 0.7), but with distribution polygons available in Roll et al. (2017), we randomly sampled 1000 presences throughout species polygon.
Environmental variablesSDMs were constructed using climatic and elevation environmental variables; however, some species were modeled using edaphic or hydrological variables, depending on their biological characteristics (i.e., aquatic, fossorial; Table S4). Thus, we initially chose 12 bioclimatic variables obtained from CHELSA v2.1 (Karger et al., 2017) and elevation (Jarvis et al., 2008), both with 1 km resolution. Edaphic variables were sourced from SoildGrid v.2.0 (Hengl et al., 2017) at 0−5 cm depth and 250 m resolution. We used Compound topographic index as a hydrological variable at 1 km resolution, as it serves as a proxy variable for watercourses (Table S4). The compound topographic index was obtained from the geomorpho90m database (Amatulli et al., 2020). All variables were upscaled to 5 km resolution with a geographic extent from the northern United States to southern South America. Initially, 17 variables were considered (Table S4). To reduce multicollinearity and the number of predictor variables, we constructed a Pearson correlation matrix (Fig. S2) using the values of the variables represented in all pixels. For all pairs of variables with a correlation ≥ |0.7|, we chose the variable with the highest biological significance. Finally, eight variables were selected (Table S4). Each species was fitted with a specific combination of variables (climatic, edaphic, hydrological, and elevation, Table S5). Although the selected variables were not correlated, the correlation structure could change for different species training areas (see species distribution models) (De Marco and Nóbrega, 2018). To overcome potential multicollinearity problems and reduce the number of predictors, we performed principal component analysis specific to each species variable combination (i.e., Table S4) and training area. Principal component analyses were performed based on correlation matrixes and selected a number of principal components that explained up to 95% of original variance (De Marco and Nóbrega, 2018). The derived principal components were used as predictors in SDMs.
Species distribution modelsWe used SDMs to estimate species distribution and habitat suitability. SDMs predict species habitat suitability and geographic distribution by relating georeferenced observations (i.e., records) with environmental predictors (Soberón et al., 2017). The SDMs were created using eight algorithms (Appendix S3). We used several algorithms because no single algorithm can deal with all modeling conditions (e.g., species prevalence, niche breadth, or records number), and allows the use of consensus models (Qiao et al., 2015). We used flexsdm v1.3.6 R package to create SDMs, which allows the creation of flexible modeling protocols, and structuring functions in pre-modeling, modeling, and post-modeling steps (Velazco et al., 2022b).
Training areas of SDMs can affect environmental quality patterns and performance metrics (Barve et al., 2011). We delimited the training area of each species using a minimum convex polygon based on species occurrences plus a buffer of 500 km. We used a technique to filter records in environmental space to reduce the sampling bias of species with > 50 records (Appendix S3). Because we did not have absence data, we randomly sampled pseudo-absences distant to 50 km from records within the training area (Appendix S3). For species with occurrences sampled from their distribution polygons, we sampled pseudo-absences outside the species polygons (Mancini et al., 2024).
It is advisable to establish different modeling protocols depending on the requirements of the species and the number of records, as these affect the distribution pattern of the species and spatial prioritization analyses for conservation (Pimenta et al., 2022). Therefore, we designed three modeling protocols established based on records number (Appendix S4; Tables S5, S6, and S7).
We used Sorensen, Area Under the Curve (AUC), and True Skill Statistic (TSS) as model performance metrics (Fig. S3). The threshold that maximizes Sorensen’s metric was used to binarize models. The final model consisted of a consensus model calculated based on the arithmetic mean of environmental suitability values. For this procedure, we used only algorithms with Sorensen values ≥ 0.7.
SDM projections over large areas can lead to overprediction of high suitability zones outside the species’ current range, impacting spatial prioritization and diversity metrics (Velazco et al., 2020). To address this, we applied a minimum convex polygon with a 100 km buffer around it (Mendes et al., 2020) and used the threshold maximizing the Sorensen metric for binary species distributions.
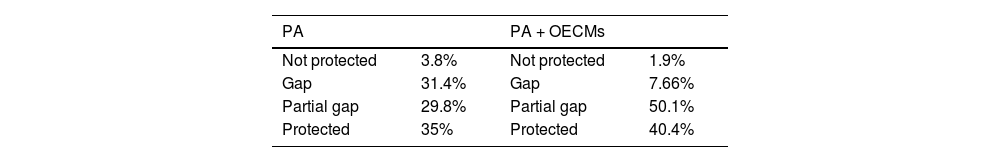
GAP analysisGap analysis assesses how well conservation areas meet representation targets for biological diversity (Rodrigues et al., 2004). Representation targets are based on species range size; species with ≤ 1000 km² require 100% of their range to be protected (i.e., within PA or OECMs), while those with ≥ 250,000 km² require 10%. Targets for species with ranges between 1000–250,000 km² were interpolated (Rodrigues et al., 2004). Species were classified as (1) 'Not Protected' if entirely unprotected, (2) 'Gap' if < 20% of the target is met, (3) 'Partial GAP' if 20-90% is met, and (4) 'Protected' if > 90% is met (Frederico et al., 2018; Table S8).
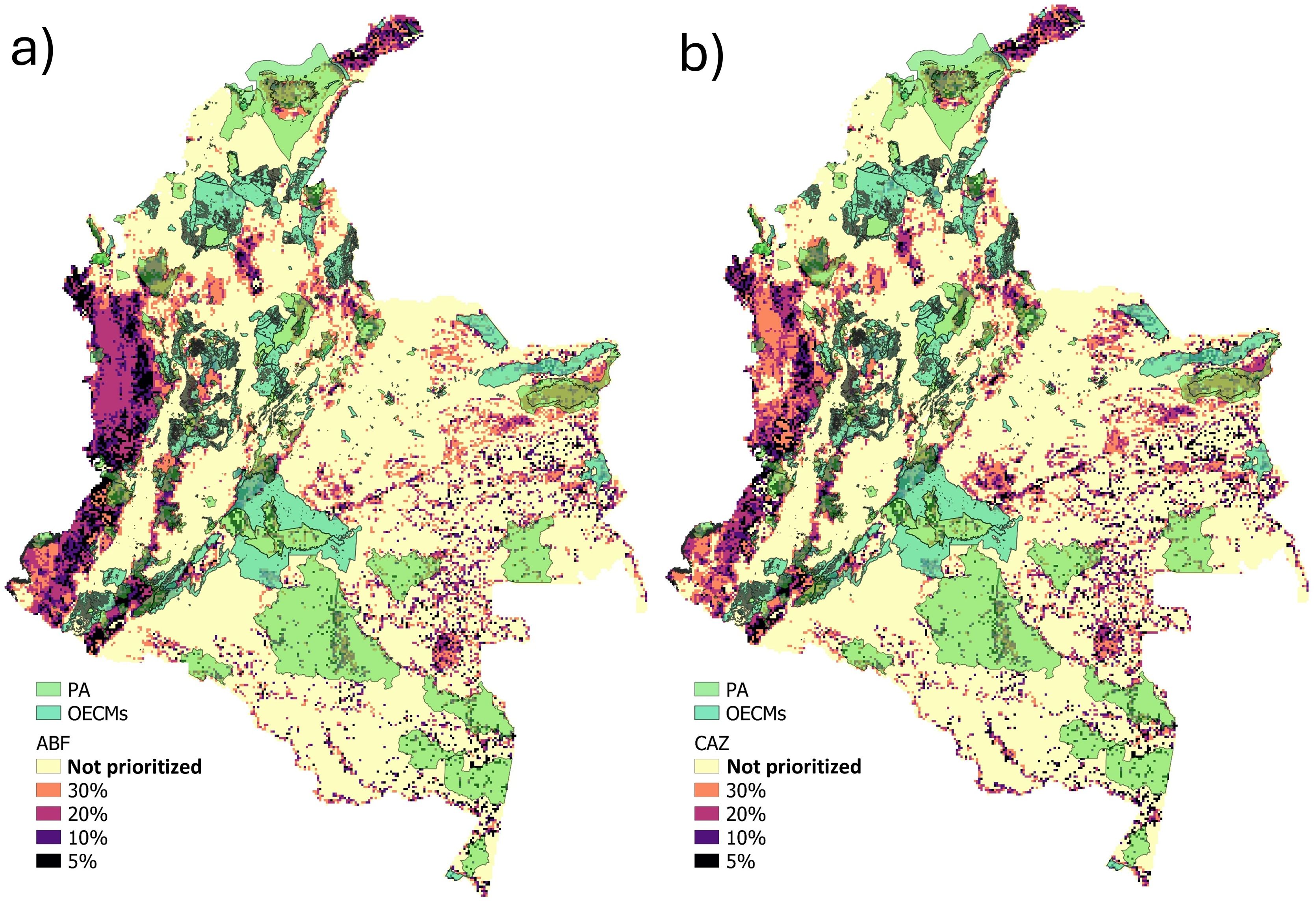
Spatial conservation prioritization and its relationship with PA and OECMsTo identify priority areas for snake conservation, we used Zonation v4 (Moilanen et al., 2014), which ranks landscape cells based on their importance for conservation using various cell removal rules, weightings, and constraints (Di Minin et al., 2014). Zonation generates a top-down ranking of all cells in the study area, based on complementarity and irreplaceability (Moilanen et al., 2005). We selected two cell removal rules, core-area Zonation (CAZ), which prioritizes cells with rare or highly weighted species, and Additive Benefit Function (ABF); which emphasizes species richness (Di Minin et al., 2014); thus, both rules provided complementary results. Species were weighted according to their degree of endemism and threat categories (Appendix S5).
We used semi-binary models (i.e., environmental suitability values greater than the threshold were kept continuous while lower ones were set to zero) to reduce the problems of inflating the spatial prioritization analysis with many cells with low environmental suitability (Domisch et al., 2019). We used habitat loss as a cost layer, calculated as the inverse of remaining habitat (Appendix S6). Prioritization solutions were categorized into four classes by selecting 5%, 10%, 20%, and 30% of the highest priority cells and calculated proportion of priority cells within PA and PA + OECMs. The selection of 30% was motivated by the 30 × 30 target (CBD, 2021).
ResultsWe modeled and estimated the distribution of 261 Colombian snake species (48 species had lower performance and were not included in our analysis). SDMs performed well for all metrics (Sorensen: 0.78 ± 0.06; AUC: 0.86 ± 0.06; TSS: 0.68 ± 0.09). For ESMs, the best-performing algorithms were BRT, MaxEnt, and ANN, with a Sorensen, AUC, and TSS above 0.7 (Fig. S3).
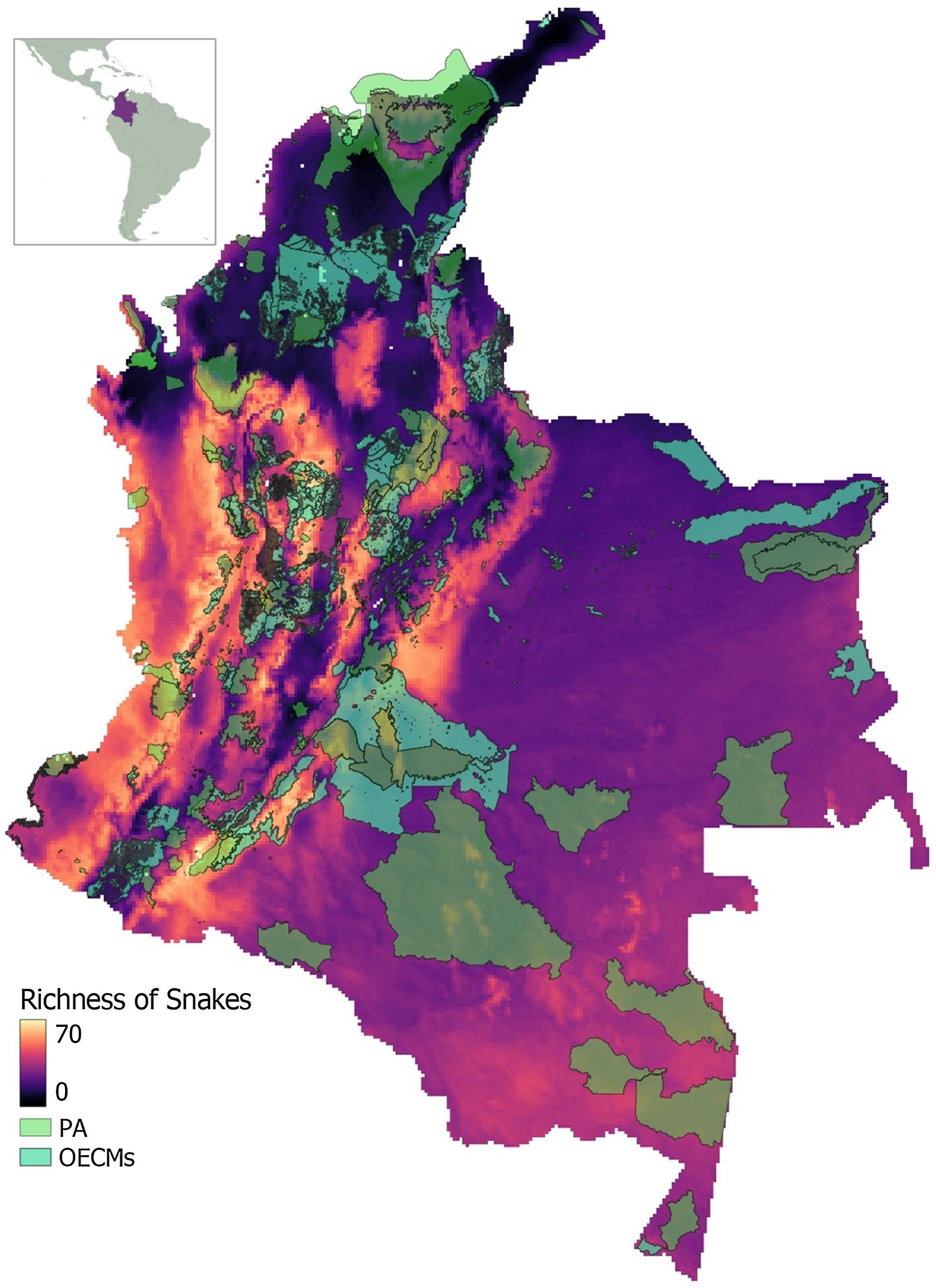
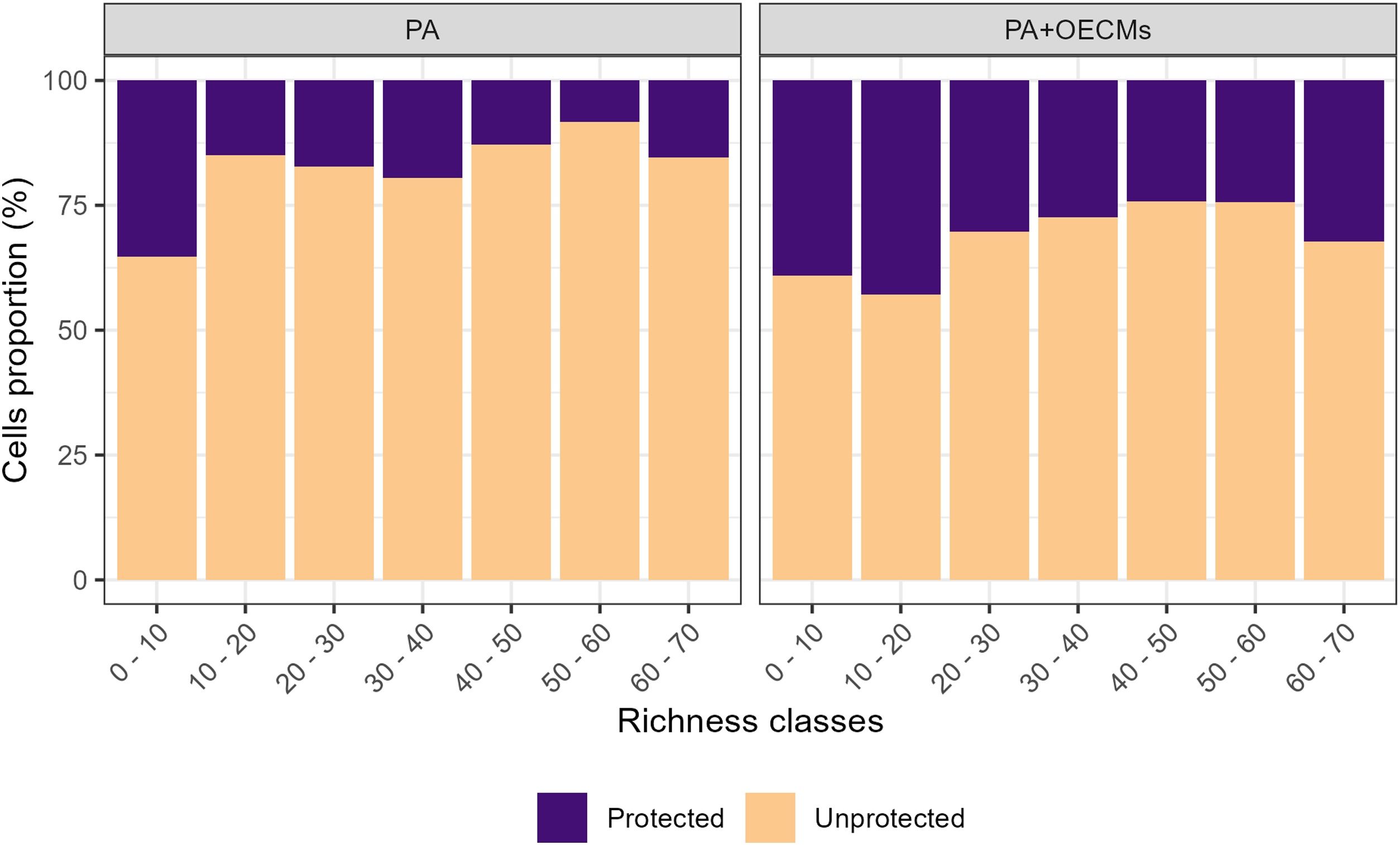
We found that species richness was the highest along mountain range slopes in the Andean, Pacific, and Amazon regions. Snake richness decreased notably in the Orinoco and most Caribbean regions (Fig. 1; Fig. S1). Regarding the relationship between species richness and PA and OECMs, only 13% of the cells with the highest species richness (>50 species) were within PA. When considering PA + OECMs, cells with the highest species richness increased slightly to 31% (Fig. 2).
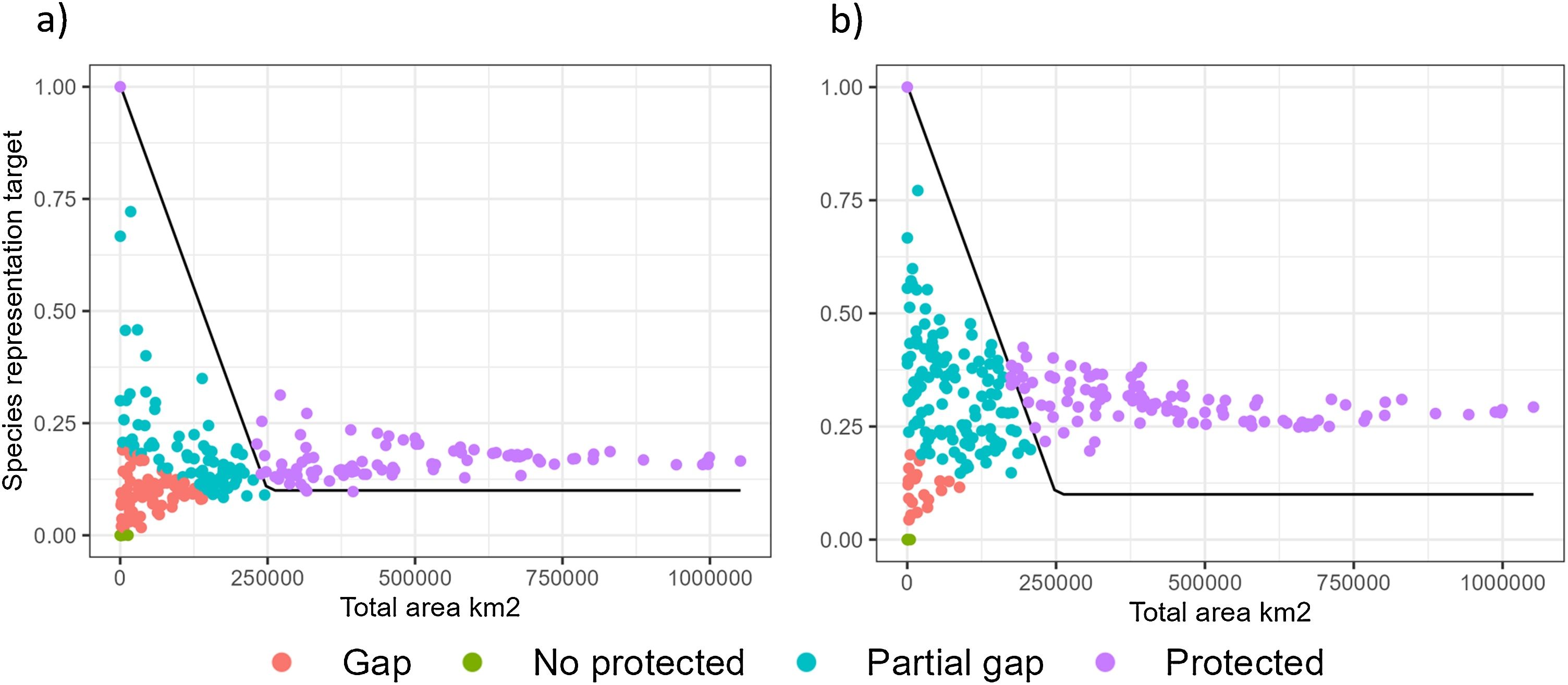
On average, snakes’ geographical ranges are 15% ± 0.09 represented within PA, rising to 29.69% ± 0.11 with PA + OECMs (Fig. S4). Gap analysis shows most species were categorized as 'Gap' or 'Protected' with PA alone, but 'Partial gap' or 'Protected' with PA + OECMs (Table 1, Fig. 3). Despite the OECMs increased the representation target already achieved by the PA (Table 1, Fig. 3), most species with distribution ranges < 125,000 km2 did not meet representation targets for either PA or PA + OECMs (Fig. 3. Table S9).
Relationship between each species' total area of distribution (km2) and the proportion achieved of the representation targets for Colombian snake species. Gap analysis with PA (a) and PA + OECMs (b). Each point represents a species, and black line represents the representation target for species distribution range size.
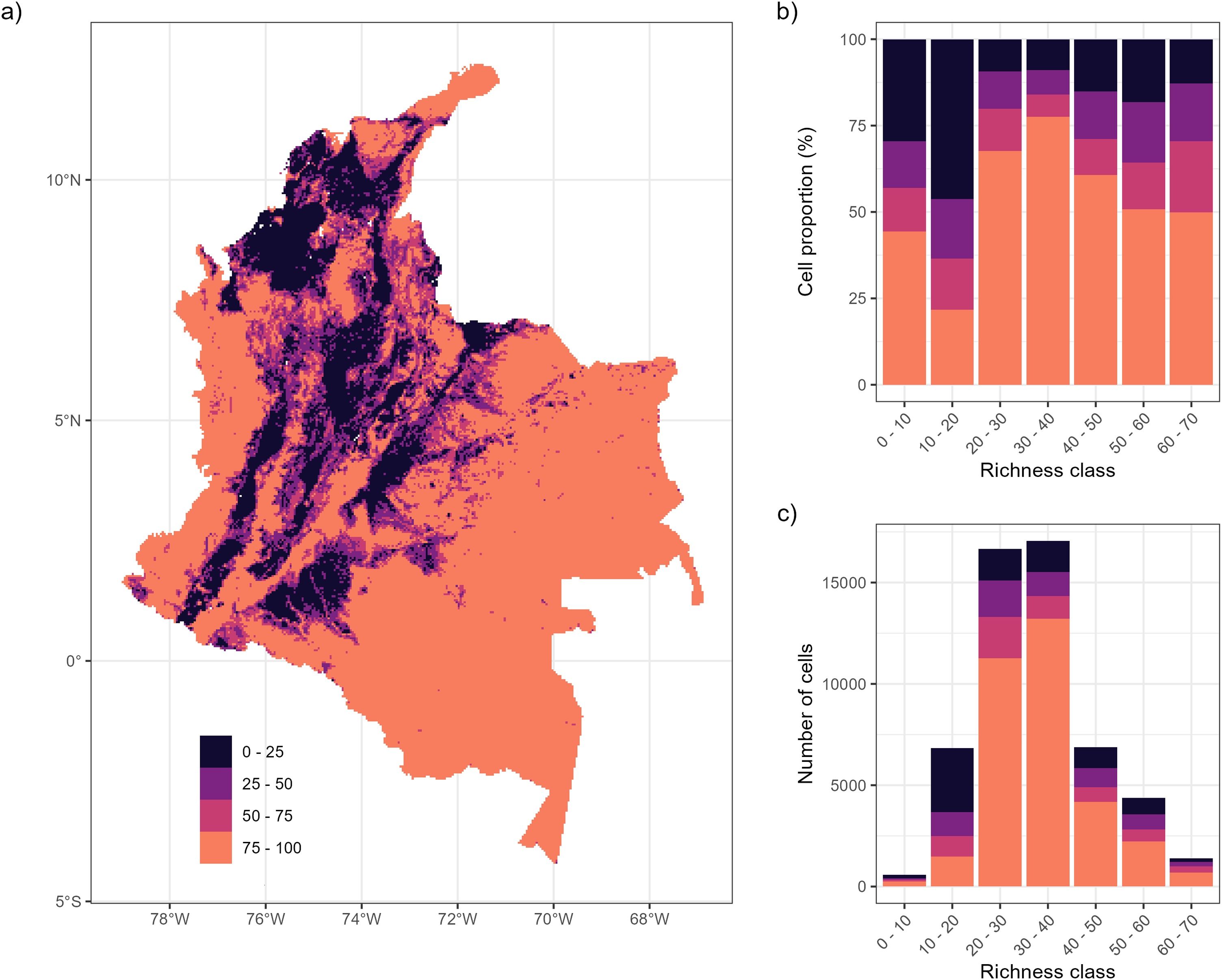
Landscapes with <50% remnant habitat represent 39.25% ± 0.08 of the study area (Fig. 4a), primarily in the northern Caribbean (except Guajira and Sierra Nevada de Santa Marta) and Andean regions (Fig. 4a). No clear pattern emerged between remnant habitat proportion and species richness (Fig. 4b-c); however, 30% with the highest species richness (50–60 and 60–70 species) overlapped with areas with < 50% of the remaining habitat (Fig. 4c). At the species level, on average snakes lost 31.16% ± 0.17 of natural cover within their ranges and 42 species experienced > 50% loss of natural cover within range.
The spatial prioritization analysis suggests that the priority areas for conservation are mainly located in the Pacific, Orinoco, Amazonia, and northern Caribbean regions (i.e., Guajira, Fig. 5). Regardless of the cell removal rule (i.e., ABF or CAZ), we found that OECMs increased the representation of priority areas. For example, the 5% priority rank, i.e., the most important cells for snake conservation, are represented by 24-27% within the PA for ABF and CAZ, and 33-37% within PA + OECMs for ABF and CAZ (Fig. S5).
DiscussionHere, we explored the relationship between snake species richness, priority areas for conservation, and representation targets with PA and OECMs for 261 Colombian species. The Andean, Pacific and Amazon regions host the highest snake richness. On average, 15% of species ranges are within the PA, but this representation doubled with OECMs. Gap analysis revealed that while OECMs increase the representation already achieved by PA, most species with a distribution range <125,000 km2 do not meet the representation target. Finally, on average, 24-27% and 33–37% of high-priority areas were within PA and PA + OECMs, respectively.
Diversity patterns in ColombiaColombia is recognized as a megadiverse territory and one of the 14 countries with the highest biodiversity worldwide (Correa, 2011). The Pacific and Andean regions had the highest snake diversity, which also stand out for their high diversity of other vertebrate groups (e.g., birds and amphibians) (Pinto-Erazo et al., 2020; Vélez et al., 2021). Although biogeographic processes explain the overall diversity of the Neotropics and the Andes (Turchetto-Zolet et al., 2013), snake richness patterns found in Colombia are understudied. We infer that snake richness in the Andean region may be driven by topographic and climatic heterogeneity that characterizes this region (Rangel, 2015, Rangel, 2010), as many studies have found a positive relationship between environmental heterogeneity and species richness (e.g., Körner, 2000; Stein et al., 2014). In contrast, high species richness in the Pacific region may be related to climatic stability (Rangel and Arellano, 2004). Pacific region has a low seasonality of precipitation and temperature, is characterized as one of the rainiest places worldwide (Rangel and Arellano, 2004) and harbors high species endemism (Rangel, 2015). Similar to other groups, the Amazon region also has considerable snake richness (Jenkins et al., 2013; Zapata-Ríos et al., 2022). It is presumed that the environmental heterogeneity associated with the formation of the Andes and the fluctuation of seasonal flooding in large alluvial river floodplains could have promoted Amazon diversity (Zapata-Ríos et al., 2022). Orinoquía and Caribbean regions exhibit lower snake richness, despite their high plant and vertebrate diversity (Jenkins et al., 2013). The lower richness towards the eastern region of Colombia appears to be consistent with other reptile and amphibian groups (IUCN, 2022a,b; Roll et al., 2017). However, the Colombian Amazon and Orinoquía present scarce species records (Suárez et al., 2021). Such data scarcity may be due to difficult access and lack of road infrastructure, in addition to being strongly affected by more than five decades of armed conflict (Cairo et al., 2018). Further studies are necessary to clarify the environmental and historical factors influencing these diversity patterns.
PA and OECMs networkColombia's PA and OECMs do not align with the highest snake richness areas, which are mostly outside these conservation networks (Figs. 1, 2). Such mismatch is consistent with amphibians and reptiles in some Colombian regions (Calderón-Caro et al., 2022; Valencia-Zuleta et al., 2014). Generally, PA suffer from different spatial bias types (Baldi et al., 2017), leading to low PA connectivity and poor representation of different ecosystems (Saura et al., 2017). Typically, PA are created in areas with touristic value, unproductive, or remote locations (Baldi et al., 2017; Phillips, 2007). Particularly Colombia's PA network started with the consolidation of some areas of economic interest, such as the Valle del Cauca, to support the sugarcane industry (Lenis, 2014). Although countries such as Colombia and Indonesia have described the limitations and challenges involved in OECMs (Atehortúa Arredondo et al., 2023; Estradivari et al., 2022), there is currently no information on their possible biases or effectiveness. However, expert consensus shed light on the opportunities and challenges of implementing OECMs (Alves-Pinto et al., 2021; Maini et al., 2023).
It has been repeatedly found that PA alone are not effective in representing biodiversity, as seen globally in mammals (Brum et al., 2017) and herpetofauna (Sánchez-Fernández and Abellán, 2015). In the case of Colombian snakes, it was no different. However, OECMs with PA doubled the representation of snake species richness. The GAP analysis showed that the categories "Partially GAP" and "Protected" also increased their representation target by 20.3% and 5.4%, respectively, for PA + OECMs. Therefore, OECMs play a crucial role in achieving conservation goals in Colombia (Rodríguez-Rodríguez et al., 2021). At the global level, OECMs can increase habitat connectivity and biodiversity representativeness (Alves-Pinto et al., 2021).
Remaining habitat and priority areasIn South America, agricultural areas have expanded rapidly over the last 30 years (Eva et al., 2004; Sy et al., 2015). For example, Brazil lost the most forest cover from 1985 to 2004, mainly due to agriculture (Sy et al., 2015; Zalles et al., 2021). Bolivia and Peru have increased the areas of anthropic uses (agriculture, livestock, and urbanization) (MapBiomas, 2023, 2024). Although Colombia created policies focused on the management and sustainable land-use (MinAmbiente, 2016, 2013), it is not different from other countries in the region, as it has lost 7.4% of its natural cover in the last three decades (Fundación Gaia Amazonas, 2023).
When exploring the patterns of the remaining habitat with the patterns of species richness, we found that the areas with the highest species richness were consistent with the areas with the lowest remaining habitat, particularly in the Caribbean and parts of the Andean region (Fig. 4a). Frequently, regions with low topographic heterogeneity, flat, coastal, and low-elevation areas have the greatest urban and agricultural development (Gao et al., 2023; Rose et al., 2023). The Caribbean region is mainly composed of low and flat lands, and ports, industrial, and agricultural activities are developed in this region (Meisel-Roca and Pérez-Valbuena, 2006). The inter-Andean valleys of the Andean region are mainly used for agriculture (DANE, 2024). Although we found no clear pattern between species richness and remaining habitat, some studies have highlighted a positive relationship (Suazo-Ortuño et al., 2008; Torres-Romero and Olalla-Tárraga, 2015), whereas others have highlighted a negative relationship between species richness and remaining habitat (Da Silva et al., 2018; Velazco et al., 2023, 2019). The presence of large regions with high species richness and high remaining habitat in Colombia suggests that many areas still hold potential for conservation efforts to protect snakes and other organism groups.
Priority areas for conservation are concentrated in the Pacific, Amazon, Orinoco, and northern Caribbean regions. Although the overlap with PA was low, it increased with the inclusion of the OECMs (Fig. S5). The largest number of communities and indigenous lands in the country are found in the northern Caribbean, Orinoco, and Amazon regions (DANE, 2012), in addition, the Pacific, Amazon, and Orinoco are regions with the highest proportion of natural cover (Fundación Gaia Amazonas, 2023). These regions represent an opportunity to identify new OECMs that, together with governmental actions will contribute to consolidating more ambitious conservation goals (Shiono et al., 2021). Biodiversity and cultural diversity are interconnected, and healthy ecosystems are the basis for the existence of indigenous peoples and local communities (Levis et al., 2024). Therefore, identifying new OECMs may represent an opportunity to transform conservation policies and practices and to recognize the contributions of indigenous peoples and local communities. (Jonas et al., 2017; Levis et al., 2024). OECMs are changing the paradigm regarding conserving biodiversity, as they generate more inclusive and representative systems that evidence multiple strategies, actors, and institutional governance arrangements at the local scale (Jonas et al., 2014).
LimitationsTo our knowledge, few studies have evaluated the contribution of OECMs to Neotropics biodiversity conservation. Unfortunately, our work suffers from some limitations; for example, gaps persist in snake records in this country, coupled with the poor model performance of some species, leading to the exclusion of 48 species from our analysis. Another limitation is that our analysis not consider the capacity of some species to persist in disturbed areas. Here, we constructed SDMs based on correlative algorithms omitting biotic interactions and species dispersal abilities that could refine distribution estimates (Soberón et al., 2017). Given the recent global implementation of OECMs, there are still no records of their long-term effectiveness (Alves-Pinto et al., 2021).
ConclusionColombia's highest snake richness regions were in the Andean, Pacific, and Amazon regions, which is consistent with other vertebrate groups. The largest areas with the least remaining snake habitats were the Caribbean and Andean regions. Based on species representativeness, Gap analysis, and overlap with priority areas, we found that OECMs contribute to the snakes' protection and complement PA. Finally, we highlight that the priority areas for conservation were concentrated in the Pacific, Orinoco, Amazon, and portions of the Caribbean regions, which are characterized by the greatest extension of natural cover, which could represent opportunities to establish new OECMs.
CRediT authorship contribution statementKGRP: Conceptualization, Software Resources, Methodology, Investigation, Data curation, Writing - Original Draft. SJEV: Conceptualization, Software Resources, Methodology, Writing - Review & Editing.
FundingKGRP was supported by PROBIU (Programa de Bolsa Institucional da Universidade Federal da Integração LatinoAmericana) scholarship. SJEV thanks FONCyT, Agencia de Ciencia y Tecnología, Ministerio de Ciencia y Tecnología de Argentina (PICTO-2022-10-00097)
Declaration of competing interestAll authors of this manuscript declare that they have no conflicts of interest that could inappropriately influence or bias the content of this research. We have no financial, personal, or professional affiliations or relationships that could be perceived as conflicts of interest in the context of this work.
Data statementOccurrences record datasets are available at doi: www.doi.org/10.6084/m9.figshare.28344461. Codes used to create species distribution models, perform spatial conservation prioritization, and perform analyses is available at doi: www.doi.org/10.6084/m9.figshare.28746371.
We thank M.B. Rose, G. Tessarolo, P. Löwenberg Neto, and M. Varajão Garey for commenting on an earlier draft of this manuscript. SJEV thanks the Center for Open Geographical Science (COGS), Department of Geography, at San Diego State University for the research support. We also acknowledge to Atlantic Forest Biodiversity Observatory from the Instituto de Biologia Subtropical (UNaM CONICET) for computing resources provided.