Land-use changes are a main driver of modifications in tropical ecosystems, leading to the loss of species and ecological traits and affecting key ecological functions. Although much attention has been given to predict the effects of species loss on ecological processes, information on the large-scale effects of land-use changes over ecological functions is scarce. Here, we detected erosion in the prevalence of ecological functions performed by mammals in response to land-use changes in the Atlantic Forest of Brazil. By analyzing the loss of different ecological functions (vertebrate and invertebrate predation, seed dispersal, seed depredation, herbivory) performed by mammal assemblages in a deforestation gradient, we observed that vulnerable functions (performed by sensitive species, such as browsing, seed depredation, medium and large vertebrate predation) were positively related to patch size and forest cover and negatively related to anthropogenic cover. These relationships were reversed for persistent functions (performed by resilient species, such as grazing, small seed dispersal, small vertebrate and invertebrate predation). Vulnerable functions were virtually restricted to large forest remnants, while persistent functions were prevalent in human-modified landscapes. Disturbed forests are not necessarily empty of mammal species, but there is a substantial loss of ecological functions across most of the Atlantic Forest. Five out of ten ecological functions lose prevalence in small forest remnants. Nonetheless, these small remnants serve as refuges for the remaining biodiversity and are on the verge of the functional extinction of important processes. The erosion of ecological functions provided by mammals compromise the persistence of Atlantic Forest's biodiversity.
Land use changes are one of the most important drivers of the loss of species and ecological traits in tropical ecosystems, particularly caused by agriculture expansion (Gibbs et al., 2010; Newbold et al., 2020). Habitat loss and fragmentation are responsible for drastic reductions in biodiversity levels, especially of large-sized fauna (Dirzo et al., 2014; Bogoni et al., 2018), which are more sensitive to land use changes. Nonetheless, most species, from small- to large-sized, threatened or not, suffer severe reductions in their populations and distribution area in response to habitat loss (Ripple et al., 2017). In the early 1990s, Redford (1992) suggested that direct and indirect effects related to habitat loss and modification, agriculture intensification and hunting, would lead to the creation of “empty forests”, resulting in the loss of significant ecological functions. Aside from the evident collapse in biodiversity and biomass (Galetti et al., 2017a), especially of large-sized birds and mammals, there are no truly “empty forests”, i.e., without animals. Thus, an important question is whether these altered assemblages in human-modified landscapes (HMLs) are able to sustain similar composition and ecological functions as assemblages in preserved areas.
As a group widely affected by land-use changes, mammals are considered a priority for conservation worldwide (Jenkins et al., 2013). Mammals are responsible for performing important ecological functions and exert a top-down effect on animal and plant populations (Ripple et al., 2014, 2015). The extirpation of large-sized mammals, such as herbivores and apex predators, may cause trophic cascade effects on ecosystems (Kurten, 2013), affecting plant recruitment and favoring rodent outbreaks and mesocarnivore populations (Terborgh et al., 2001; Estes et al., 2011). Thus, increasing knowledge of functional changes in mammal assemblages between preserved areas and HMLs is necessary for ecosystem management and conservation planning, aiming for the maintenance not only of animal populations but also of ecological functions.
The Atlantic Forest in South America used to be one of the largest tropical and subtropical rainforests in the world, ranging from 3 to 30 degrees latitude (Ribeiro et al., 2009). Despite being a biodiversity-rich biome, most of its extent was replaced by monodominant agriculture and pastures, and the remaining forest remnants are small and immersed in anthropogenic matrices (Ribeiro et al., 2009). This landscape structure directly affects the occurrence and persistence of species, acting as a selective filter that eliminates sensitive species and modifies assemblage composition (Prugh et al., 2008).
The Atlantic Forest presents an interesting study case regarding the maintenance of ecological functions performed by its fauna, since there is a broad variation in the structure and composition of its landscapes. This biome sustains a high diversity of mammals (Abreu et al., 2020) that varies from preserved areas to HMLs, allowing investigation of whether ecological functions are maintained, are reduced in prevalence or disappear altogether in response to land use changes. We defined the prevalence of an ecological function in a local community as the proportion of those species that perform the focal ecological function out of all species present in the mammal assemblage.
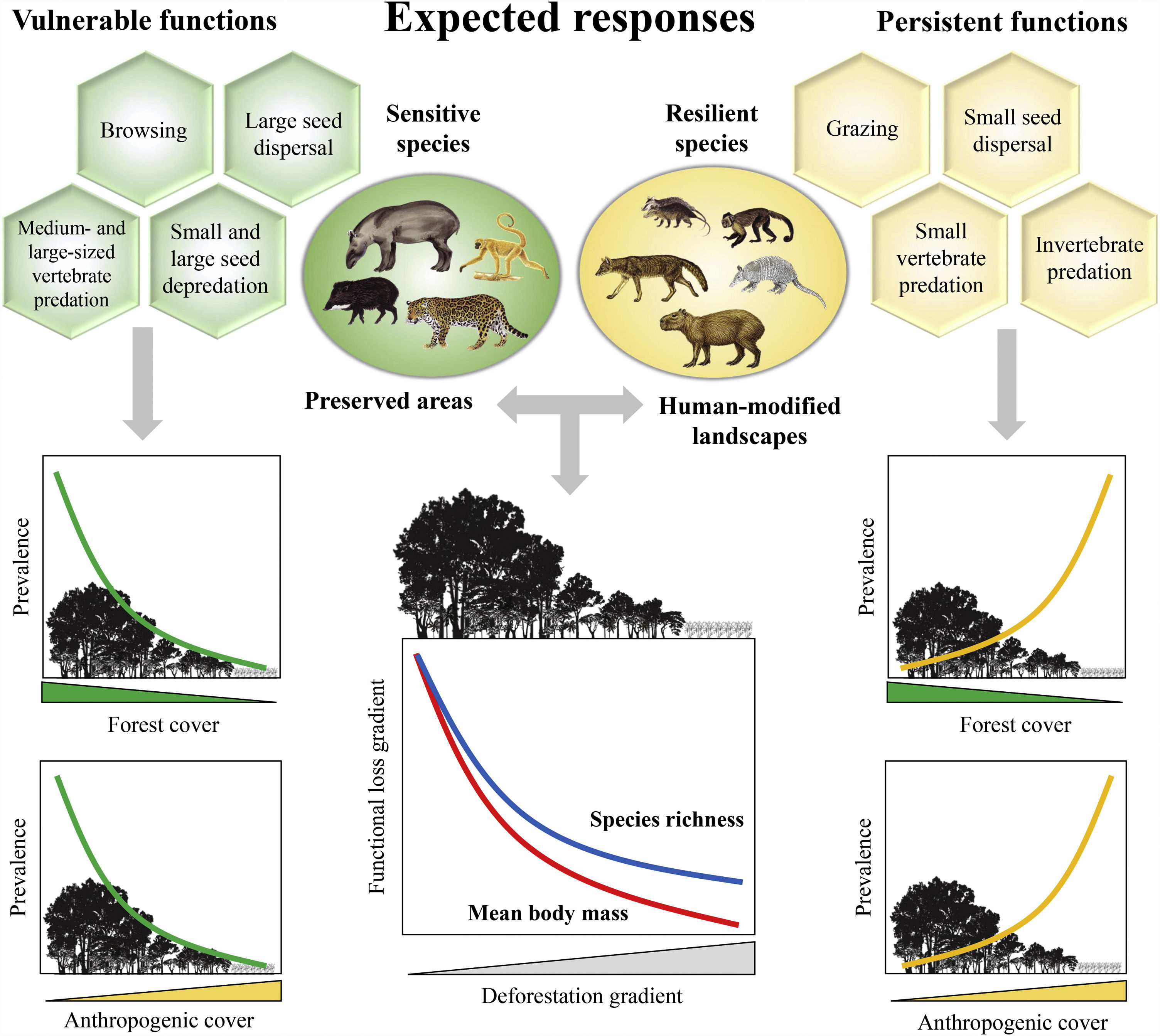
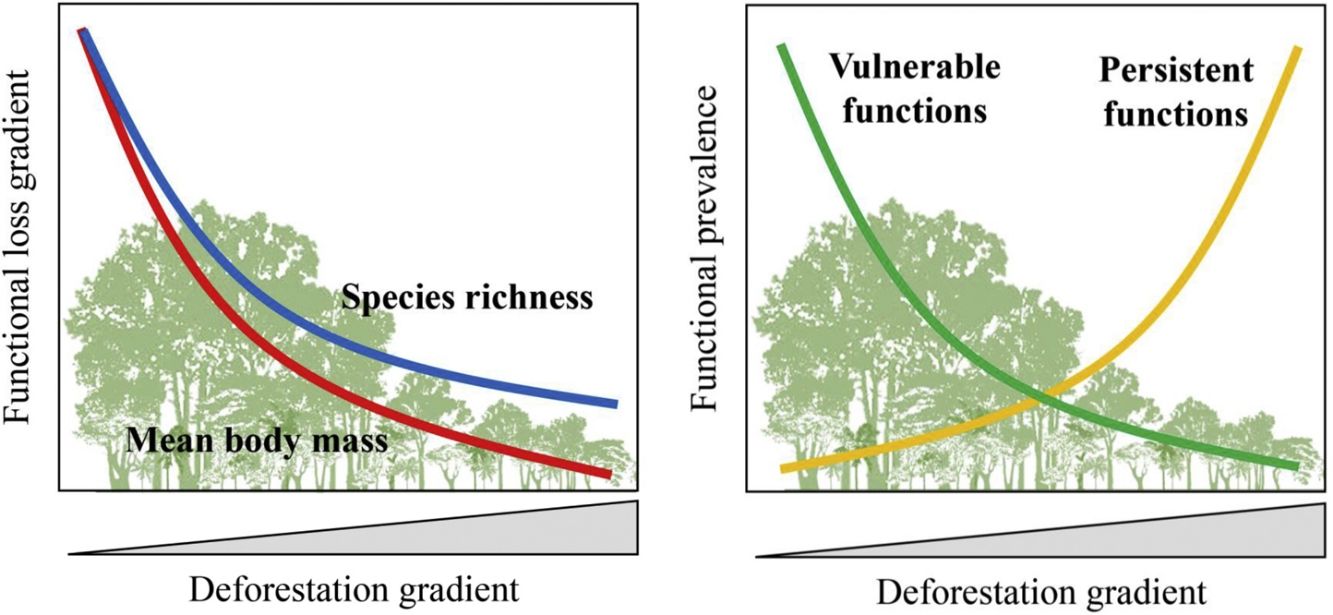
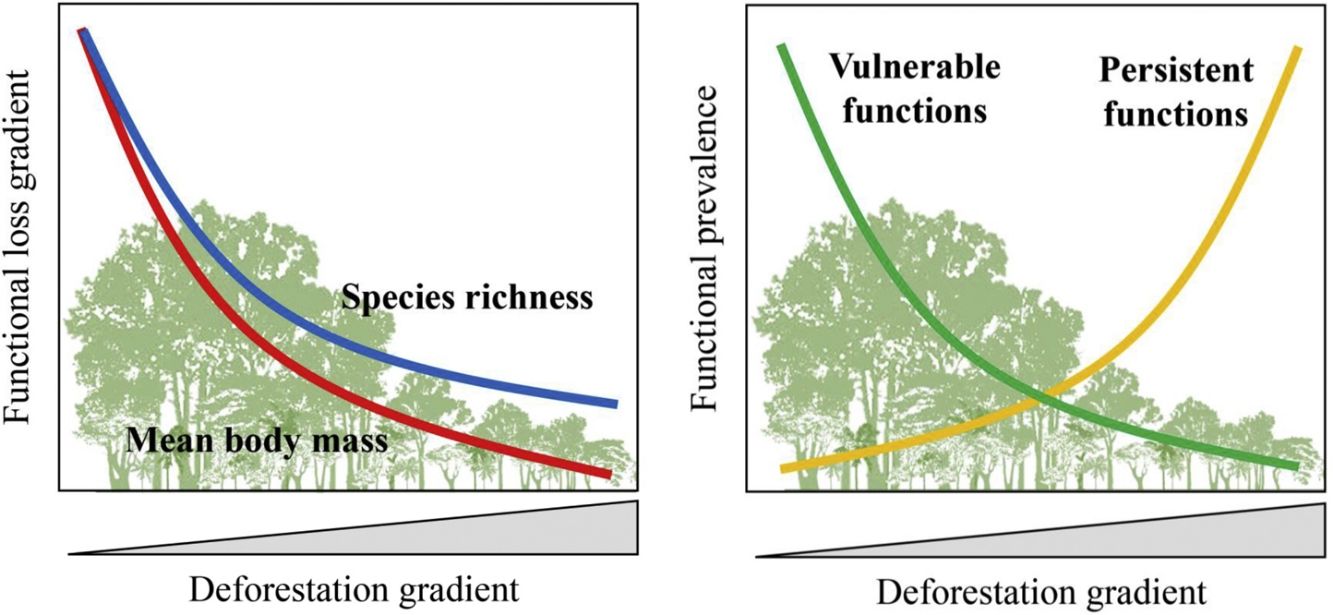
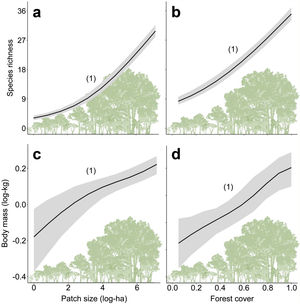
Furthermore, there are still some knowledge gaps regarding ecological functions, such as what is the relationship between species loss and maintenance of ecological functions? (Isbell et al., 2018). Understanding how land-use changes affect biodiversity and their related ecological functions and services is essential to produce effective measures for their conservation (Mitchell et al., 2015). Thus, the objective of our study was to quantify changes in the prevalence of ecological functions, mean body mass and species richness of medium- and large-sized mammal assemblages in response to land-use changes in the Atlantic Forest of Brazil. Species richness and body mass are strongly related to ecological functions (Cardinale et al., 2006; Hector and Bagchi, 2007; Isbell et al., 2011), and thus, good predictors of whether the deforestation gradient translates into a gradient of functional loss (Fig. 1).
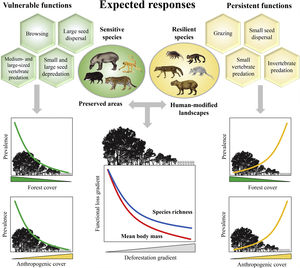
Expected patterns for the ecological functions. Vulnerable functions (i.e., those performed by large-sized species and species sensitive to habitat loss) such as browsing, large seed dispersal, small and large seed depredation, medium- and large-sized vertebrate predation, are negatively affected by reductions in patch size and forest cover and increasing anthropogenic cover. Conversely, these factors benefit species that are resilient to habitat loss and environmental modifications, increasing the prevalence of persistent functions, such as grazing, small seed dispersal, and small-sized vertebrate and invertebrate predation. The pattern of the ecological functions will follow the decrease in assemblage species richness and mean body mass as deforestation increases.
Based on literature, we classified ecological functions into vulnerable and persistent. We defined vulnerable functions as those performed by large-sized species and species sensitive to habitat loss, such as browsing, seed depredation, large seed dispersal, and medium- and large-sized vertebrate predation (Redford, 1992; Wright, 2003; Terborgh et al., 2008). The functions performed by species resilient to habitat loss and modified habitats, such as grazing, small seed dispersal, small-sized vertebrate and invertebrate predation, were defined as persistent functions. Therefore, we expect that the prevalence of vulnerable functions will decrease in response to a decrease in forest cover and an increase in anthropogenic cover; the opposite pattern is expected for persistent functions (Fig. 1).
Materials and methodsDatasetsFirst, we created a dataset of the composition of mammal assemblages encompassing a deforestation gradient of the Atlantic Forest (details on Appendix 1 and Appendix 2 – Tables S1 and S2); then, based on the literature, we created another dataset composed of studies on the diet/feeding habits of mammals in the biome, which was used as a proxy to measure their contribution in performing each function (details on Appendix 1 and Appendix 2 – Table S3). For both datasets, we performed a literature search in Web of Science and Google Scholar using different combinations of keywords in English and Portuguese. All studies used for analysis that composes each dataset are properly cited in Appendix 2 – Tables S1 and S3.
For the assemblage dataset, we selected 63 studies of medium- and large-sized mammals within the Atlantic Forest, resulting in 96 assemblages (Appendix 1 and Appendix 2 – Table S1 and S2), of which we extracted the total species richness and presence/absence data, and calculated the average body mass of each assemblage for analysis.
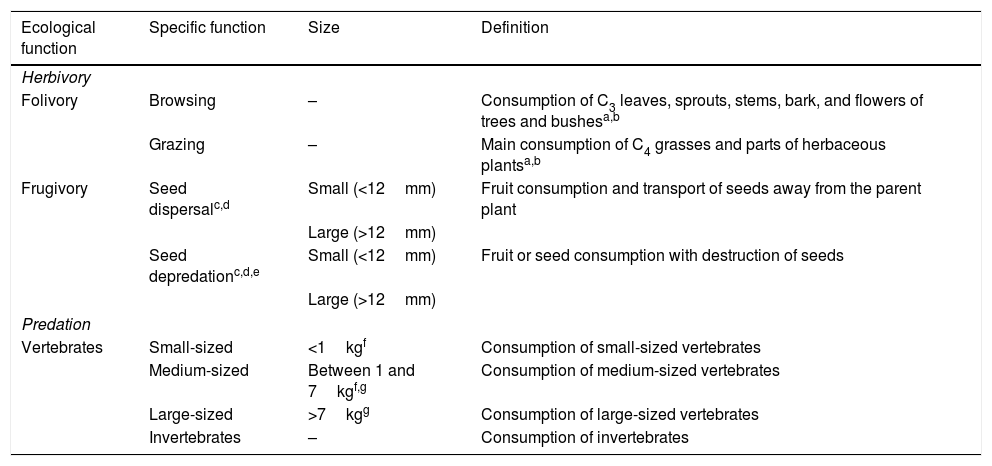
For the diet dataset, we first selected two main ecological functions as bases – herbivory (folivory and frugivory) and predation (vertebrates and invertebrates) – which we divided into 10 specific functions for analysis (Table 1). To determine the contribution of mammals in performing each function, we selected 454 diet/feeding habit studies for all species present in the assemblage dataset (N=83; Appendix 1). Then, from these studies we extracted the relative percentage (0–100%) of food items composing the species diet, corresponding to the ecological functions selected for analysis, and used this percentage as a proxy of their contribution to each function. For each ecological function performed by the mammals, we presented a mean value and a standard deviation (Appendix 2 – Table S3).
Main ecological functions – herbivory and predation – divided into 10 specific functions performed by medium- and large-sized mammals of the Atlantic Forest, Brazil.
| Ecological function | Specific function | Size | Definition |
|---|---|---|---|
| Herbivory | |||
| Folivory | Browsing | – | Consumption of C3 leaves, sprouts, stems, bark, and flowers of trees and bushesa,b |
| Grazing | – | Main consumption of C4 grasses and parts of herbaceous plantsa,b | |
| Frugivory | Seed dispersalc,d | Small (<12mm) | Fruit consumption and transport of seeds away from the parent plant |
| Large (>12mm) | |||
| Seed depredationc,d,e | Small (<12mm) | Fruit or seed consumption with destruction of seeds | |
| Large (>12mm) | |||
| Predation | |||
| Vertebrates | Small-sized | <1kgf | Consumption of small-sized vertebrates |
| Medium-sized | Between 1 and 7kgf,g | Consumption of medium-sized vertebrates | |
| Large-sized | >7kgg | Consumption of large-sized vertebrates | |
| Invertebrates | – | Consumption of invertebrates | |
aHofmann and Stewart (1972); bChapman and Reiss (1999); cSeed thresholds obtained from Bello et al. (2015); dData on seed diameter was obtained from Bello et al. (2017); eInformation on seed depredation was obtained from studies in the diet dataset (Appendix 2 – Table S3); fAdapted from Chiarello (2000); gAdapted from Emmons and Feer (1997).
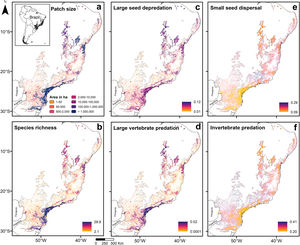
To test how land use change affects the prevalence of ecological functions performed by the mammal assemblages, we generated five landscape variables using the MapBiomas land use map (Projeto MapBiomas, 2017), collection 2 of 2016, with spatial resolution of 30m: patch size and percentages of forest cover, pasture, urban area, and small-scale mosaics of mixed land use classes (henceforth called mixed land use). We considered as anthropogenic cover: pasture, urban area and mixed land use. Landscape variables calculated in 2-km buffers for medium- and large-sized mammals present the strongest relationships with explanatory variables, as noted by some studies (e.g., Lyra-Jorge et al., 2010; Beca et al., 2017). Yet, to statistically justify the use of this buffer size, we calculated our variables in buffers with radii of 0.5, 1, 2, 5 and 10km for the 96 study sites and then fitted five multiple regression models, one for each buffer size, comparing assemblage's species richness with landscape variables (Appendix 3 – Table S4). Variables (land use classes) calculated in 2-km buffers presented the most significant relationship with species richness (Adjusted R2=0.41; F=20.33; p<0.001).
To be able to extrapolate the results for the entire Atlantic Forest based on the relationships obtained for the 96 assemblages analyzed (based on a fitted statistical model; see the section Statistical analyses), we calculated the same landscape variables using the MapBiomas land use map for the entire biome.
For the calculation of landscape variables, we used the ‘LandScape Metrics’ package (https://github.com/LEEClab/LS_METRICS), available in GRASS GIS 7.2.2. To do this, we first reclassified the MapBiomas land use map into several binary maps (one for each land use class), where the value 1 refers to the focal class (i.e., forest, pasture, urban area, and mixed land use), and the value 0 (zero) refers to other classes. Using these binary maps, we calculate the variables using the search radius of 1-km and, lastly, we resampled the final raster maps to a 1-km resolution. Therefore, we had a set of 1-km spatial resolution raster maps with pixel values referring to patch size and cover percentages of each land use classes.
Statistical analysesWe analyzed the data statistically with Hierarchical Modelling of Species Communities (HMSC) (Ovaskainen et al., 2017), an approach that belongs to the class of joint species distribution models. We fitted a multivariate probit regression model, where the response variable was the presence or absence of the 83 mammal species in the 96 study sites (Appendix 2 – Tables S1 and S2). As explanatory variables, we used the log-transformed patch size, and the other landscape variables (forest cover, pasture, mixed land uses and urban areas), and as traits, we included the log-transformed body mass and the 10 ecological functions of the diet dataset (Table 1; Appendix 2 – Table S3). To account for spatial autocorrelation in the data and to enable spatial predictions, we included in the model as a random effect, spatially explicit latent factors (Ovaskainen et al., 2016) that model residual variation in the occurrences and co-occurrences of the species (not explained by the covariates included in the model). We assumed the default prior distributions of Ovaskainen et al. (2017) and sampled the posterior distribution with 10,000 Markov Chain Monte Carlo (MCMC) iterations, of which we ignored the first 3,000 as transients. We followed Ovaskainen et al. (2017) to perform a variance partitioning between the explanatory variables and the random effects implemented through the spatial latent factors.
We conducted a set of scenario simulations to examine how the functional composition of the assemblages depends on environmental predictors. In these simulations, we considered one of the environmental predictors (e.g., patch size) as the focal predictor, the value of which we varied with 10 uniformly distributed values from the smallest to the largest observed in the data. We generated 100 simulated assemblages for each value that we assumed for the focal predictor. When generating predictions, we set the values of the nonfocal environmental predictors to their mean value conditional on the value of the focal predictor. We computed for each of the simulated assemblages its species richness, the mean body mass, and the proportion of species that belonged to each ecological function (i.e., the functional prevalence). We further characterized the variation in assemblage similarity along these gradients by computing the similarity of each focal assemblage to the assemblages generated at the two extremes (lowest and highest values) of the environmental gradient. We summarized the results based on the posterior mean and 95% credible intervals.
To create extrapolated maps of species richness and community-weighted mean traits, we used the fitted HMSC model to predict the occurrence probabilities for each species for the entire Atlantic Forest biome. These predictions were based on the distributions of the predictor variables calculated for the entire biome (see the section Landscape metrics), and they are based both on the estimated fixed effects (patch size and land use classes), and on the spatial latent variables, which allow for interpolation in the neighborhood of the observed data and thus improve the predictive performance (Ovaskainen et al., 2016). We summarized the predicted assemblages in terms of their species richness, mean body mass and prevalence of ecological functions. A schematic illustration of the statistical analyses performed by HMSC is shown Appendix 3 – Fig. S2. Graphical implementation was done using the package ‘ggplot2′ (Wickham, 2016) available in R 3.6.3 (R Core Team, 2020).
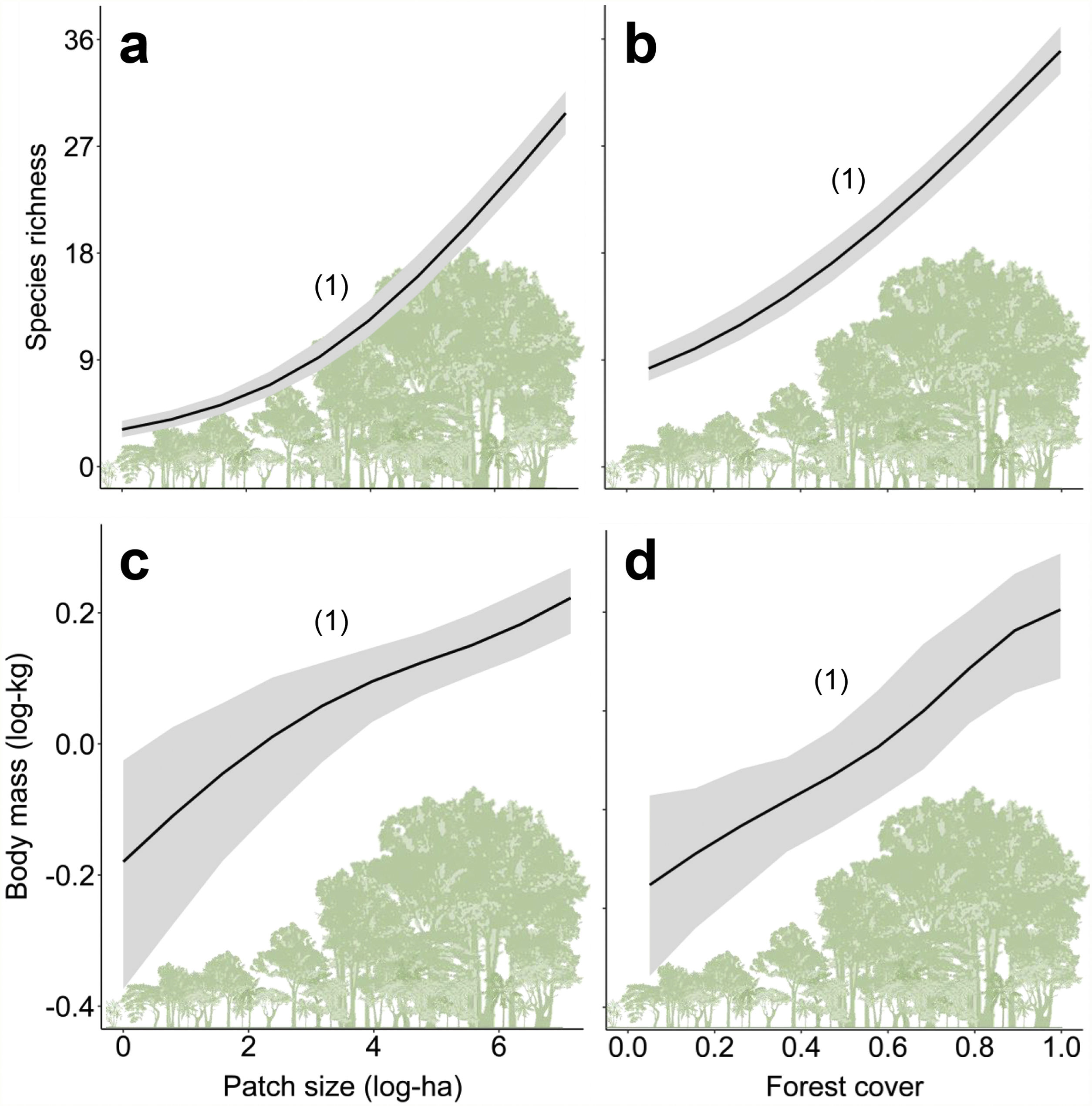
ResultsPrevalence of ecological functions over environmental gradientsThe average explanatory power over the species was 0.27 in units of Tjur (2009) R2, and thus the model predicted occurrence probabilities were on average 0.27 higher for occupied sites than for unoccupied sites. As expected, the rarest species were the most difficult to model (Appendix 3 – Fig. S3). Partitioning the variables that explained the variation in assemblage composition (HMSC output), patch size (47%) was the most important, followed by land use class (38%) and random effects (14%); this and other outputs of the model are in Appendix 3 – Figs. S3–S7. Assemblage species richness and mean body mass were positively related with patch size and forest cover and had a negative influence of anthropogenic cover (Table 2; Fig. 2; Appendix 4), characterizing gradients of species and ecological traits loss in response to deforestation.
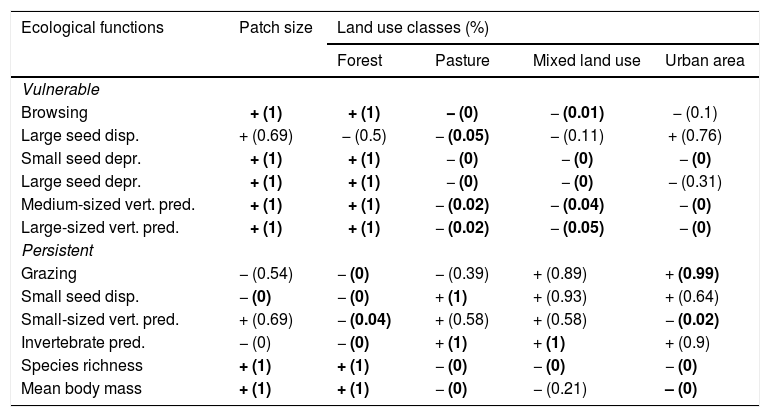
Positive or negative relationships between species richness, mean body mass and ecological functions performed by assemblages of medium- and large-sized mammals of the Atlantic Forest, Brazil, and landscape variables (see also Appendix 4). The numbers in brackets give the posterior probability by which the community-weighted mean value for each ecological function is greater at the largest value of the environmental variable than at the smallest value of the environmental variable. The posterior probabilities are rounded to two decimals, so that “1” means “>0.995” and “0” means “<0.005”. For example, the value “1” for “Browsing” versus “Patch size” means that there is very strong statistical support (posterior probability>0.995) for the proportion of species following the browsing strategy being larger in large patches (more precisely, patches as large as the largest in our data) than in small patches (more precisely, patches as small as the smallest in our data). Cases with at least 0.95 posterior support are highlighted in bold, +/− indicate a positive or negative correlation between two variables, respectively.
| Ecological functions | Patch size | Land use classes (%) | |||
|---|---|---|---|---|---|
| Forest | Pasture | Mixed land use | Urban area | ||
| Vulnerable | |||||
| Browsing | + (1) | + (1) | − (0) | − (0.01) | − (0.1) |
| Large seed disp. | + (0.69) | − (0.5) | − (0.05) | − (0.11) | + (0.76) |
| Small seed depr. | + (1) | + (1) | − (0) | − (0) | − (0) |
| Large seed depr. | + (1) | + (1) | − (0) | − (0) | − (0.31) |
| Medium-sized vert. pred. | + (1) | + (1) | − (0.02) | − (0.04) | − (0) |
| Large-sized vert. pred. | + (1) | + (1) | − (0.02) | − (0.05) | − (0) |
| Persistent | |||||
| Grazing | − (0.54) | − (0) | − (0.39) | + (0.89) | + (0.99) |
| Small seed disp. | − (0) | − (0) | + (1) | + (0.93) | + (0.64) |
| Small-sized vert. pred. | + (0.69) | − (0.04) | + (0.58) | + (0.58) | − (0.02) |
| Invertebrate pred. | − (0) | − (0) | + (1) | + (1) | + (0.9) |
| Species richness | + (1) | + (1) | − (0) | − (0) | − (0) |
| Mean body mass | + (1) | + (1) | − (0) | − (0.21) | – (0) |
Relationships between species richness and mean body mass with patch size (a and c) and forest cover (b and d) for the assemblages of medium- and large-sized mammals of the Atlantic Forest, Brazil. Solid black lines represent the posterior mean and the grey areas the 95% credible intervals of the model predictions. The numbers in brackets give the posterior probabilities of the Bayesian regressions. The posterior probabilities are rounded to two decimals, so that “1” means “>0.995” and “0” means “<0.005”).
Similarly, all ecological functions were mainly affected by forest cover, patch size and pasture, reflecting their importance in shaping assemblage composition and the prevalence of the ecological functions (Table 2; Appendix 4). Except for large seed dispersal, vulnerable functions increased with patch size and forest cover – the same trend found for species richness and body mass (Table 2; Appendix 4). Persistent functions, in turn, decreased with at least one of patch size or forest cover. Persistent functions also increased with anthropogenic cover (Table 2; Appendix 4). The results partially comply with our expectations, showing that most functions performed by sensitive species and large-sized species declined with changes in landscape structure, while those performed by resilient species increased in prevalence (Fig. 1). Nevertheless, grazing, large seed dispersal and small-sized vertebrate predation were poorly explained by landscape variables (Table 2; Appendix 4 – Figs. S11, S12 and S15).
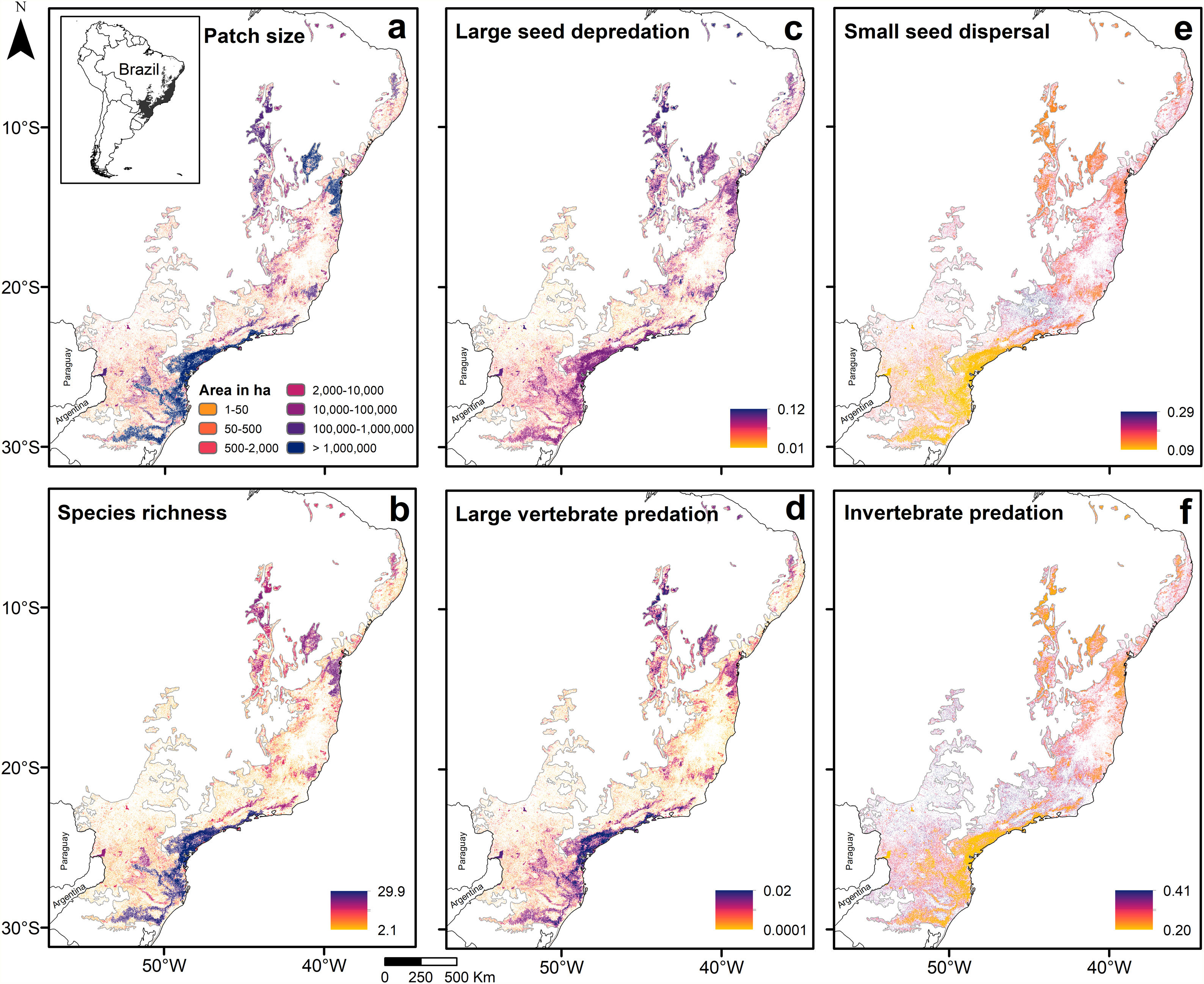
Predictions across the Atlantic ForestPredictions for the entire biome (Fig. 3; Appendix 5) were consistent with the analysis of prevalence and in accordance with expected responses (Fig. 1). Predictions for species richness were strongly and positively related to patch size (Fig. 3a and b), the same occurring to the mean body mass (Appendix 5 – Fig. S22). Vulnerable functions, such as large seed depredation (Fig. 3c) and large-sized vertebrate predation (Fig. 3d), had similar responses and were more prevalent in large forest remnants. Conversely, persistent functions, such as small seed dispersal (Fig. 3e) and invertebrate predation (Fig. 3f), showed the opposite pattern and were more prevalent in small forest remnants within HMLs. Functions such as browsing, grazing and small-sized vertebrate predation were more influenced by assemblage composition at lower latitudes (<20° S), presenting patterns that slightly diverged from our predictions. Lastly, large seed dispersal had predictions contrary to our expectations (Appendix 5 – Fig. S26), reflecting the results of the analysis of prevalence.
DiscussionThe loss of species and ecological traits, resulting from habitat loss, patch isolation and fragmentation, was responsible for the decrease in the prevalence of five out of ten functions, translating a deforestation gradient into a functional loss gradient and corroborating the need for high biodiversity levels to maintain ecological functions and ecosystem services (Loreau et al., 2001; Oliver et al., 2015). Vulnerable functions (i.e., performed by large-sized species and species sensitive to habitat loss) were prevalent in large and continuous forest remnants, highlighting the irreplaceability of primary forests for maintaining ecological functions (Gibson et al., 2011; Watson et al., 2018). In ecosystems where large-sized herbivores and apex predators are extirpated, cascading effects are expected, impacting plant and vertebrate populations, favoring mesocarnivores and small-sized generalists, and affecting ecosystem processes such as carbon storage and nutrient cycling (Terborgh et al., 2001; Estes et al., 2011; Ripple et al., 2014, 2015). The impacts aforementioned reflect the situation for persistent functions (i.e., functions performed by species resilient to habitat loss and modified habitats), which were more prevalent in HMLs of the Atlantic Forest. Benefiting from anthropogenic cover, resilient species are present in most of the assemblages, resulting in a high prevalence of functions such as small seed dispersal and predation of small-sized vertebrates and invertebrates in HMLs.
Species richness and mean body massMammal assemblage composition was mostly explained by patch size and forest cover, which are strong predictors of species richness and diversity (Banks-Leite et al., 2014), functional diversity (Bovo et al., 2018a; Magioli et al., 2015, 2016) and the dynamics of ecological processes (Dobson et al., 2006). Body mass is an important predictor of species sensitivity to habitat loss and can be related to ecological functions they perform (Brose et al., 2005; Brown et al., 2004). In fact, the abundance of mammal functional groups is differently affected by land use changes, being more intense for large-sized species (Newbold et al., 2020). Species richness and body mass are strongly associated with ecological functions and ecosystem services (Cardinale et al., 2006; Hector and Bagchi, 2007; Isbell et al., 2011), and their elevated values in large forest remnants highlight the importance of preserving these areas.
Vulnerable functionsVulnerable functions were more prevalent in large and continuous forest remnants, browsing, for example, clearly presented this pattern. Browsers exert a top-down effect on plant species, influencing their abundance, recruitment and diversity (Cook-Patton et al., 2014). Areas rich in browser species have elevated functional redundancy, which may augment the prevalence of a function. The key mammal browsers in the Atlantic Forest [i.e., large-sized primates (Brachyteles spp., Alouatta spp.), sloths (Bradypus spp.), brocket deer (Mazama spp.) and lowland tapir (Tapirus terrestris)] are typically sensitive to habitat loss and restricted to large remnants, which explains the prevalence of this function in large remnants (Appendix 5 – Fig. S23). The low prevalence of browsing in HMLs is in accordance with the global reduction of large-sized herbivores (Ripple et al., 2015), that has resulted in the loss of functional redundancy, which negatively affects ecological functions and ecosystem functioning (Reich et al., 2012), and may worsen the loss of plant richness and diversity in HMLs (Ripple et al., 2015). This reduction also increases due to the preference of hunters in some regions of the Atlantic Forest for brocket deer and lowland tapirs (Cullen et al., 2000, 2001; Chagas et al., 2015).
The context is similar for seed depredation, which apparently exerts a more significant top-down effect on plant population size than browsing does (Maron and Crone, 2006). Large-sized depredators, such as peccaries (Tayassu pecari and Pecari tajacu) and brocket deer, exert a competitive buffer effect on small-sized species (<1kg), preventing rodent outbreaks and intense seed depredation that may reduce plant diversity (DeMattia et al., 2004; Dirzo et al., 2007). The predictions for seed depredation (Appendix 5 – Figs. S27 and S28) support this cause-effect relationship, i.e., high prevalence in large and continuous remnants and low prevalence in HMLs. This context suggests that in HMLs, small mammals are promoting intense seed removal (Culot et al., 2017), especially of small seeds (Dirzo et al., 2007), reducing plant diversity, and consequently affecting other ecological processes. This pattern is reinforced by the hunting preference for large seed depredators such as peccaries, brocket deer, lowland paca (Cuniculus paca) and agoutis (Dasyprocta spp.) across the Atlantic Forest (Cullen et al., 2000, 2001; Castilho et al., 2017; Sousa and Srbek-Araujo, 2017), further reducing their contribution in HMLs.
In the Atlantic Forest, two main species perform predation on medium- and large-sized mammals, pumas (Puma concolor) and jaguars (Panthera onca). Jaguars are apex predators in the biome, but their population has drastically declined (Galetti et al., 2013); currently, jaguar populations are isolated and restricted to a few large forest remnants (Paviolo et al., 2016), and subject to retaliatory hunting (Marchini and Macdonald, 2012). The loss of large-bodied species occupying high trophic levels compromises the prevalence of the functions they perform (Dobson et al., 2006; Duffy, 2003) and causes cascading effects that impact ecosystem functioning (Estes et al., 2011; Terborgh et al., 2001). Other sensitive predators (e.g., Pteronura brasiliensis, Speothos venaticus) have practically disappeared from the Atlantic Forest (Nagy-Reis et al., 2020), further reducing the prevalence of their functions, except in large and continuous remnants, in accordance with previous studies showing that carnivores were more intensely affected by land use changes (Newbold et al., 2020).
Contrary to our expectations, large seed dispersal was poorly explained by patch size and forest cover. Out of 29 species performing this function, only four have a high contribution [three agouti species and lowland paca (Appendix 2 – Table S3)]. However, these species are relatively common in HMLs (see studies in Appendix 2 – Tables S1 and S2), which could explain the higher prevalence of large seed dispersal in small forest remnants. In addition, omnivores that benefit from HMLs (e.g., canids and procyonids; Beca et al., 2017; Bovo et al., 2018b; Magioli et al., 2016), despite presenting a low contribution to this function (<10%), might help increase the prevalence of large seed dispersal in these areas. Species considered important dispersers of large seeds such as muriquis (Brachyteles spp.) and lowland tapirs (Bueno et al., 2013), are rare and restricted to few large and continuous Atlantic Forest remnants (Jorge et al., 2013), where they are also subject to hunting (Galetti et al., 2017a), which may explain the low prevalence of large seed dispersal in large remnants. Nonetheless, we emphasize that information on the consumption of fruits with large seeds by medium- and large-sized mammals and the capability of species in dispersing these seeds is limited, which may have caused a bias in our results. Studies aiming at increasing knowledge on the natural history of extant large seed dispersers, especially feeding habits (e.g., observational studies, isotopic ecology), to reduce this bias.
Persistent functionsFunctions such as grazing, which are performed by resilient species, presented the reverse pattern compared to those seen in sensitive species, showing high prevalence in HMLs. There are few grazer species in the Atlantic Forest, mainly because of the habitat type, i.e., rainforests are different from savannas, where grazers are dominant herbivores. However, currently, most of the biome is dominated by agriculture and pastures, with anthropogenic cover accounting for ∼64% of its original domain (Projeto MapBiomas, 2017). This landscape composition benefits species such as capybaras (Hydrochoerus hydrochaeris) (Bovo et al., 2016), the largest grazer in Brazil. The difference in species composition along the Atlantic Forest explains the variation in grazing prevalence at higher latitudes (>20° S) (Appendix 5 – Fig. S24) because above this threshold capybaras are more abundant and other grazer species are present [e.g., the coypu (Myocastor coypu)].
Small seed dispersal was more prevalent in HMLs and favored by higher proportion of pasture in the landscape. Primates are the main performers of this function (∼45% of all species; Appendix 2 – Table S3), but this taxonomic group is severely threatened with extinction both in Brazil and worldwide (ICMBio/MMA, 2018; IUCN, 2020), and several species have restricted distributions [e.g., tamarins (Leontopithecus spp.) and muriquis; Culot et al., 2019]. Nevertheless, while primate richness is reduced in HMLs, opossums and seed dispersing carnivores (i.e., canids, procyonids and mustelids) are more abundant. This situation is corroborated by the presence of species tolerant of open habitats [e.g., maned wolf (Chrysocyon brachyurus), white-eared opossum (Didelphis albiventris)], which are absent from large forest remnants. This change in assemblage composition increases the prevalence of small seed dispersal in HMLs (Appendix 5 – Fig. S25).
Despite the evident loss of species with deforestation, functions such as small-sized vertebrate predation are still prevalent in small forest remnants, mainly because carnivores, particularly mesocarnivores, persist. Small-sized vertebrates, such as small mammals (<1kg), are the resource baseline for most mesocarnivores (Verdade et al., 2011). In HMLs, small mammals present low species richness, but some generalist species of rodents and marsupials might increase in abundance (Bovendorp et al., 2017; Figueiredo et al., 2017). The abundance of these species linked with the availability of food resources in the agricultural matrix (i.e., crops) can increase the support capacity of HMLs (Verdade et al., 2011), benefiting mesocarnivores. The presence of species tolerant to open habitats and their plasticity in thriving in modified landscapes (Magioli et al., 2014, 2019) explains the higher prevalence of small-sized vertebrate predation in HMLs (Appendix 5 – Fig. S29).
Invertebrate predation presented the clearest and strongest patterns in relation to landscape variables, being negatively impacted by patch size and forest cover and benefitted by anthropogenic cover. The main invertebrate predators in HMLs are resilient species that are tolerant of open habitats and agricultural areas, such as armadillos (Dasypus novemcinctus and Euphractus sexcinctus), opossums (Didelphis spp.) and procyonids (Nasua nasua and Procyon cancrivorus) (e.g., Beca et al., 2017; Bovo et al., 2018b; Magioli et al., 2016; Magioli et al., 2019). Although the richness of invertebrates such as ants is low in HMLs (Martello et al., 2018), this context favors generalist and omnivore species, which increase in abundance. The fact that most invertebrate predators persist in HMLs of Atlantic Forest, and are present in most of the assemblages in these landscapes (Appendix 2 – Tables S1 and S2), corroborates our predictions for the high prevalence of this function across HMLs of the biome (Appendix 5 – Fig. S32).
Challenges of accounting other factors and limitationsWe recognize that there are limitations in using proxies to investigate ecological and ecosystem processes (e.g., Hatfield et al., 2018), similarly to the diet composition dataset we used in this study. There are many approaches available that can be used to relate functional traits to ecological/ecosystem processes (Mouchet et al., 2010). Here we chose the HMSC approach over other functional diversity metrics as we were interested in predicting how each ecological function related to land-use variables and functional diversity approaches would mask this information. Despite a few exceptions, most of the functions we analyzed presented strong relationships and biologically meaningful patterns with land-use variables.
There is a myriad of other factors that may also influence the prevalence of ecological functions performed by mammals, such as hunting pressure. Despite the limitation of not including hunting pressure or other factors as variables in our model, our findings were well explained by landscape composition and presented strong relationships, also agreeing with previous results on defaunation patterns for the Atlantic Forest (Canale et al., 2012; Galetti et al., 2017a) and across the Neotropics (Bogoni et al., 2020). As case study, we work only with medium- and large-sized mammals and observed more variation in the prevalence of some ecological functions than expected (e.g., small seed dispersal). This variation can be attributed to the role played by other taxa performing the same function, which could balance and/or compensate this variation, such as the role of birds in the case of small seed dispersal.
Implications for conservationThe Atlantic Forest small remnants may not be “empty forests” (sensu Redford, 1992), but are being emptied of the functions the species perform, losing half of the ecological functions due to the loss of mammals. Five out of ten ecological functions disappear in small forest remnants. In the Atlantic Forest, small remnants (<2500ha) account for ∼65% of all native forest remaining in the biome (Ribeiro et al., 2009). Our study highlights that large remnants of primary forest are irreplaceable for maintaining biodiversity and its functions, as remnants of structurally complex vegetation sustain more functions performed by mammals (Sukma et al., 2019). Some of the most important remnants of primary forest are under legal protection in the biome (e.g., Serra do Mar State Park with >1Mha), but recent evidence shows that even protected areas are under intense human pressure (Jones et al., 2018) and that there is low connectivity among them (Ward et al., 2020). Therefore, the remaining large remnants and the current protected area network may not be enough to maintain, in the long-term, the myriad of ecological functions performed by mammals in the Atlantic Forest.
Small forest remnants serve as refuges for the remaining biodiversity, but sustain few ecological functions. Nonetheless, these small remnants still play a role of extreme importance by serving as a baseline for ecological restoration, as stepping stones to create structural connections between large remnants and protected areas (Wintle et al., 2019). These may also be target for refaunation initiatives, aiming to restore diverse biomes such as tropical rainforests (Galetti et al., 2017b). As the Atlantic Forest is pinpointed as a priority for restoration (Strassburg et al., 2020), our predictions for the ecological functions serve as basis to indicate areas where to direct investments for restoration, not only halting species loss but also contributing to the maintenance of vulnerable ecological functions.
FundingThis work was supported by the São Paulo Research Foundation (FAPESP) [grant numbers 2014/09300-0, 2014/10192-7, 2016/19106-1]; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001; Fundação Grupo Boticário de Proteção à Natureza [grant numbers 201410014, 201710015]; National Council of Technological and Scientific Development (CNPq) [grant numbers 308503/2014-7, 308632/2018-4]; Academy of Finland [grant numbers 284601, 309581, 308651]; Research Council of Norway [grant number 223257]; ERKKO foundation (Research Center for Ecological Change).
Declaration of interestsNone.
We are grateful to the Forest Science Department (“Luiz de Queiroz” College of Agriculture, University of São Paulo), the Interdisciplinary Graduate Program in Applied Ecology (PPGI-EA) and the Wildlife Ecology, Management and Conservation Lab (LEMaC). We thank Renata Pardini and Ronaldo G. Morato for the comments and suggestions on an early version of this manuscript.
The following are the supplementary data to this article:
Relationships between species richness, mean body mass and the prevalence of the 10 ecological functions performed by assemblages of medium- and large-sized mammals of the Atlantic Forest, Brazil, and landscape variables (patch size, and percentages of forest cover, pasture, mixed land use and urban areas). (Figs. S8–S19).