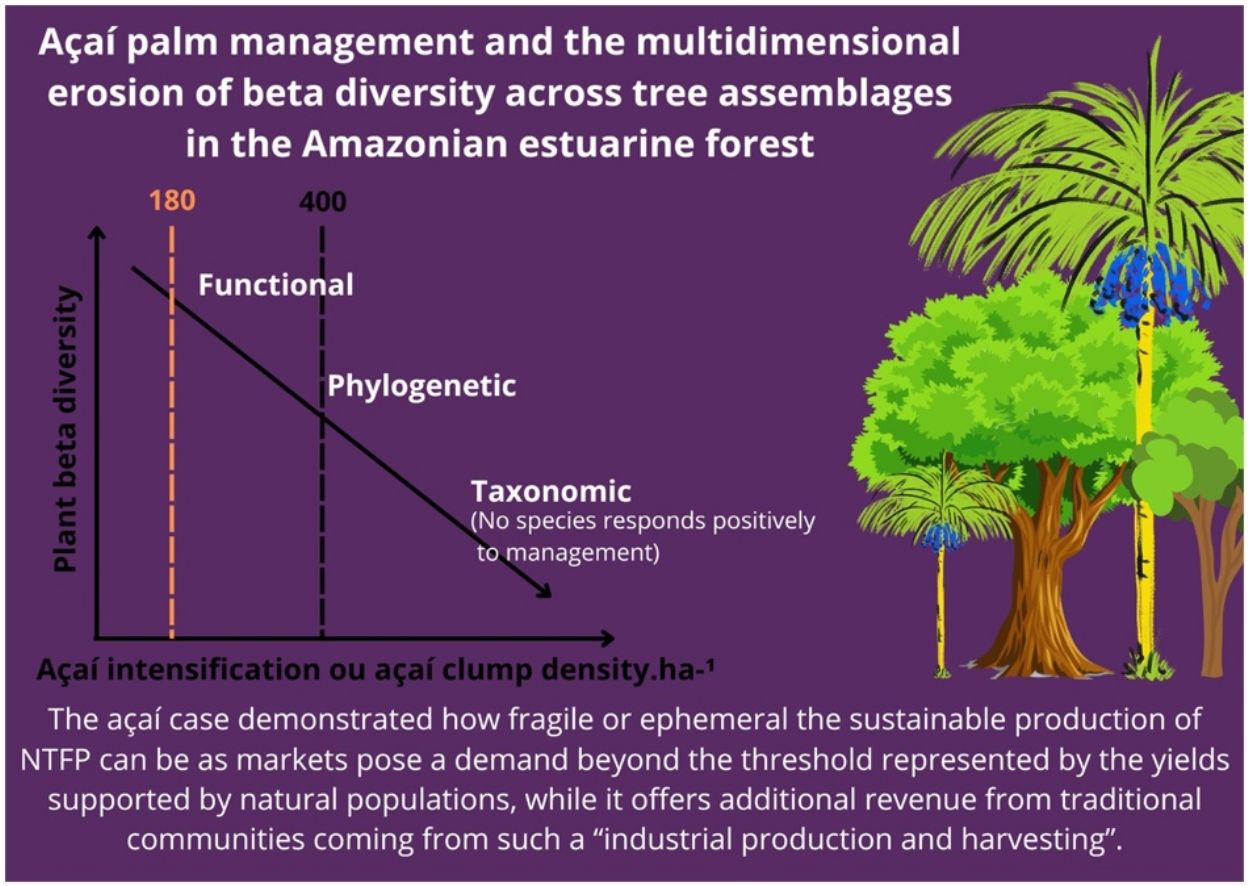
Non-timber forest products persist as an opportunity to conciliate tropical forest integrity and better life for traditional communities, but sustainability must be continuously evaluated. This paper examines diversity-related impacts from increments on the açaí palm density for fruit production (i.e., açaí intensification) on the tree assemblages of the Amazon estuarine forest. By examining 43 forest stands covering 20–1260 açaí clump.ha−1, we documented a decline on taxonomic, phylogenetic and functional beta diversity, which resulted from species loss along the intensification gradient. Such an impoverished community assembly resulted from the fact that forest stands with <400 açaí clump.ha−1 exhibited higher scores of species accumulation or beta diversity, while no species responded positively to increments on açaí clump density and 17 species did negatively. The community-level threshold for species loss was 180 clump.ha−1, and after the 400-clumps threshold (as posed by current regulation) almost half of the tree species was already lost. Our results suggest that the açaí intensification represents a driver of tree species assembly and a tangible threat for integrity of the Amazon estuarine forest by promoting a multidimensional community impoverishment at regional scale. Threat magnitude depends on which extension managed, high-density açaí stands replace forest patches supporting açaí natural densities. The açaí case demonstrated how fragile or ephemeral the sustainable production of non-timber forest products can be, since markets pose a demand beyond the threshold represented by the yields supported by natural populations, while it offers additional revenue from traditional communities coming from such a “industrial production and harvesting”.
The exploitation of non-timber forest products (hereafter NTFPs) continues to gain relevance as an instrument to enhance tropical forest use, able to guarantee forest integrity and the myriad of ecosystem services provided from biodiversity conservation to climate regulation (Gaoue and Ticktin, 2008; Nadkarni and Kuehl, 2013). More than a source of revenue for traditional communities (i.e., forest dwellers) based on local markets or a driver for regional economies, NTFPs are now integrated into complex approaches such as (1) the restoration of commercial logging forests (Cerullo and Edwards, 2019), (2) biocultural keystone species for ecosystem functioning (Shackleton et al., 2018), (3) an instrument to preserve cultural values and heritages (Pradhan and Ormsby, 2020), (4) a component of forest biocultural restoration (Gavin et al., 2015), (5) food security and social reproduction of traditional cultures (Cocks et al., 2011), and (6) bioeconomy-based regional development (Costa et al., 2021). Such a broad perspective is to expand as some NTFPs continue to achieve global markets and thus offer opportunities for socioeconomic development and forest persistence and integrity, since extraction does not overtake the ecological sustainability thresholds (Gaoue and Ticktin, 2008; Mello et al., 2020).
We already know that the harvesting of NTFPs is able to impact tropical forests at multiple levels of ecological organization, from populations to ecosystem level, although the majority of studies have focused on species/population level (Gaoue et al., 2013, 2014; Hart-Fredeluces et al., 2022; Ticktin, 2004). Recent findings have reported impacts on plant-animal interactions (Campbell et al., 2022), tree (Freitas et al., 2021) and regenerating (Barros et al., 2023) assemblage organization and ecosystem-level attributes such as ecosystem productivity, nutrient storage, aboveground biomass and biological invasion (Eddy and Basyuni, 2020; Keet et al., 2023; Kull and Shackleton, 2023; Ruwanza and Shackleton, 2017). Even considering a relatively reduced number of studies addressing both community- and ecosystem-level impacts, it is possible to conclude that the effects depend on harvest intensity (Gaoue and Ticktin, 2008; Ndangalasi et al., 2007). Although in most cases harvesting has been considerable sustainable (Hernández-Barrios et al., 2015; Hidalgo Pizango et al., 2022), sustainability is context-dependent and a fragile achievement as harvest intensity respond to a myriad of drivers, including market demand (Mello et al., 2020; Hernández-Barrios et al., 2015).
Sustainability as a fragile achievement appears to be is the case of the açaí fruit harvesting in the estuarine forest of the Amazon region; i.e., an evergreen floodplain forest. Fruits of this canopy palm species (Euterpe oleracea Mart.), which is native and occurs naturally across some Amazon floodplain forests, has been historically used to prepare a beverage consumed daily by locals (i.e., traditional riverine populations) as a staple food (Brondízio, 2008). From a traditional use, the açaí fruit has gained urban consumers globally as a component of food items and nutritional supplements such as energetic drinks and ice-creams, with a superfood status (Magrach and Sanz, 2020). Açaí is now present across four continents and more than 40 countries (IBGE, 2020; Tavares et al., 2020). An increasing demand for açaí fruits has driven locals to increase açaí palm density across forest stands via management, including the elimination of undesired trees to favor açaí palm recruitment, growth and fruit production by reducing competition, particularly for light (Freitas et al., 2015; Weinstein and Moegenburg, 2004). Forest stands naturally supporting <100 açaí clump.ha−1 have been pushed to over 600 clumps.ha−1 via management (Freitas et al., 2015, 2021). Such an intensification (hereafter açaí intensification) has provided considerable gains of productivity at household level supporting a considerable socioeconomic development across the estuarine region with a central benefit for the local agro-extractivism communities (Brondízio, 2008; Vogt et al., 2016; Weinstein and Moegenburg, 2004). By harvesting 1.3 million fruit tons annually, the açaí regional revenue has achieved over a 1-billion R$ year and favored an immense number of locals (IBGE, 2020; Tavares et al., 2020), which are directly involved in the açaí fruit chain (IBGE, 2020). Accordingly, açaí has been referred as the “Amazon black gold” (Costa et al., 2021), but also represents a “case” in the context of the NTFP-related promises.
In fact, açaí intensification started in the 80’s and it is now a regional phenomenon. However, açaí intensification has demonstrated to reduce tree abundance and species richness of estuarine forest patches (Freitas et al., 2021), with understory tree species emerging as a sensitive group (Barros et al., 2023), and pose negative impacts on regenerating assemblage (Barros et al., 2023) and seed rain and soil seed bank (Silva et al., 2023). By locally reducing tree species richness by 10–80%, via non-random stem elimination, it is reasonable to propose that açaí intensification promotes drastic changes in tree assemblages at landscape and regional level. In fact, as tree species richness declines at stand level, açaí intensification would alter patterns of beta diversity (i.e., accumulation of biological information through de space), considering the taxonomic, phylogenetic and functional dimensions, since species extirpation is not expected to occur randomly in tropical human-modified landscapes (Filgueiras et al., 2021). Among several possibilities, intensification can promote either (1) forest/biotic homogenization through decreasing beta diversity and increments on community-level similarity (Castro Solar et al., 2015; Olden and Rooney, 2006; Püttker et al., 2015), (2) diversification through increments on beta diversity with a decrease in species similarity (Socolar et al., 2016), or (3) increments on community nestedness in the case species poor assemblages become a subset of richer assemblages (Baselga, 2010; Socolar et al., 2016), leading to community impoverishment at multiple spatial scale as already proposed (Chase et al., 2019). These potential forest trajectories or transitions go beyond local impoverishment of tree assemblages with unanticipated impacts on forest integrity and, consequently, in the perspective of a sustainable açaí production by managing native populations across Amazonian floodplain forests and its socioecological corollary.
Here, we test the hypothesis that açaí intensification reduces tree-assemblage beta diversity considering the taxonomic, phylogenetic and functional dimensions in the Amazon estuarine forest, the current core area of açaí fruit production. Although previous findings have already documented significant drops in taxonomic alpha diversity at particular sites (Freitas et al., 2021; Barros et al., 2023), beta diversity at regional spatial scale and potential processes such as biotic homogenization and community nestedness have never been examined considering taxonomic, phylogenetic and the functional dimension of the native assemblages. We refer to a potential regional-wide forest degradation in addition to other current drivers such as logging, edge effects, intense droughts and wildfires (Lapola et al., 2023). Diversity scores from 47 tree assemblages spread across the region were contrasted to clump.ha−1. Moreover, clump density thresholds are associated with expressive decline on tree species richness, but also with the taxonomic and ecological groups which decline as açaí clump density increases. Our uncovered patterns are discussed in the light of underlying mechanisms leading to forest impoverishment, their consequences, lessons and potential mitigation guidelines.
Material and methodsStudy regionThe Amazon estuarine region consists of a large floodplain belt exposed to the tide regime imposed by the Atlantic Ocean in the eastern Amazon region. This estuarine habitat covers nearly 376,000 km2 by embracing continental areas and a myriad of fluvial islands formed by the sediments from the Amazon river and some tributaries such as the Tocantins and Guamá rivers (Fig. S1). The climate is hot and humid (Af, Kottek et al., 2006), with mean temperatures between 26 °C to 27 °C and mean annual precipitation achieving 2800−3200 mm across the region, ranging from 32 mm and 465 mm throughout the year (Instituto Nacional de Meteorologia - INMET, 2023). The estuarine plain is covered by an evergreen floodplain forest, which is exposed to a tide regime supporting daily/monthly flooding events, particularly during the high tides (Junk et al., 2020). A small extension of the estuarine plain is probably exposed to the long-term annual flooding supported by the Amazon river (Pires and Prance, 1985). Available information indicates that this forest is highly diverse considering both tree and shrub species across multiple spatial scales (Freitas et al., 2015, 2021; Barros et al., 2023). We shall mention the occurrence of sumaúma (Ceiba pentandra (L.) Gaertn., Malvaceae), one of the largest tropical tree species, which offers a typical physiognomy for the estuarine forest and its emergent layer (Anderson et al., 1994).
Historically, this region has been occupied by small-holding riverine rural communities with livelihood based on the exploitation of forest products (timber and NTFPs), fishery and slash-and-burn agriculture (Brondízio, 2008; Vogt et al., 2016). The exploitation of açaí fruits for producing the açaí beverage has been part of the riverine culture (the caboclos) as the beverage represents a stapple food. Naturally, açaí palms occur as clumps with 20–210 clumps per hectare (Freitas et al., 2015, 2021). In order to obtain larger yields and thus meet a growing market demand, locals have increased açaí clump density up to 1260 clump.ha−1 through the expense of tree assemblages via forest thinning. Tree stem reduction is required to give space and offer more illuminated habitats for açaí clumps (Freire et al., 2013; Homma et al., 2006). Forest thinning usually spare useful plants, mainly cocoa (Theobroma cacao Mar., Malvaceae), ucuuba (Virola surimaensis (Rol.) Warb. (Myristicaceae) and andiroba (Carapa guianensis L., Meliaceae), while other forest stands are completely converted into açaí monoculture (Moegenburg and Levey, 2002; Pollak et al., 1995; Vogt et al., 2015; Weinstein and Moegenburg, 2004).
Tree assemblage datasetOur dataset from tree species assemblages were provided by previous studies (Freitas et al., 2015, 2021; Table S1) summing up 47 forest 0.1-ha plots across seven municipalities (Belém Metropolitan Region, Barcarena and Abaetetuba) in the estuarine region (Fig. 1, Table S1). This dataset was intentionally built to (1) cover the entire range exhibited by the açaí palm density in the estuarine forest, from natural stands (<200 clumps per ha) up to forest stands managed to achieve high palm densities (Freitas et al., 2015), and (2) cover as much as possible the estuarine region. Otherwise, it could not be possible to test the effects of the intensification process as already done for smaller spatial scales relative to alpha diversity (Freitas et al., 2021; Barros et al., 2023). We shall acknowledge that forest stands supporting natural or low açaí palm density are already rare in the region, thus drastically limiting our possibilities relative to plot location. However, here we did not assume that our range described the frequency through which managed forest stands occur in the region, although intensification is a very common and spreading practice (Barros et al., 2023). All trees (DBH ≥ 5 cm) across 0.1 ha plots were sampled. Sample coverage was between 80% and 90%, indicating that the sampling effort was sufficient to estimate the plant species richness across forest stands (Chao and Jost, 2012). Moreover, this plot size permitted recording between 108 and 1265 stems. Thereby we considered 0.1 ha as a proper plot size. Species were identified at the field site and by comparison in herbarium MG of the Museu Paraense Emílio Goeldi, with help of an expert para-taxonomist, following the APG IV classification (Chase et al., 2016). In the study forest stands mean açaí palm density varied from 20 to 1260 clump.ha−1 (mean of 392,8 ± 338.3 clump.ha−1). In total, we recorded 2804 trees belonging to 203 species and 46 families. The most species-rich families were Fabaceae (30% of species), followed by Malvaceae (10%) and Meliaceae (7%). Finally, our dataset is available in https://data.mendeley.com/datasets/7k3ky2vwck/1, Mendeley data published on 5 August 2024.
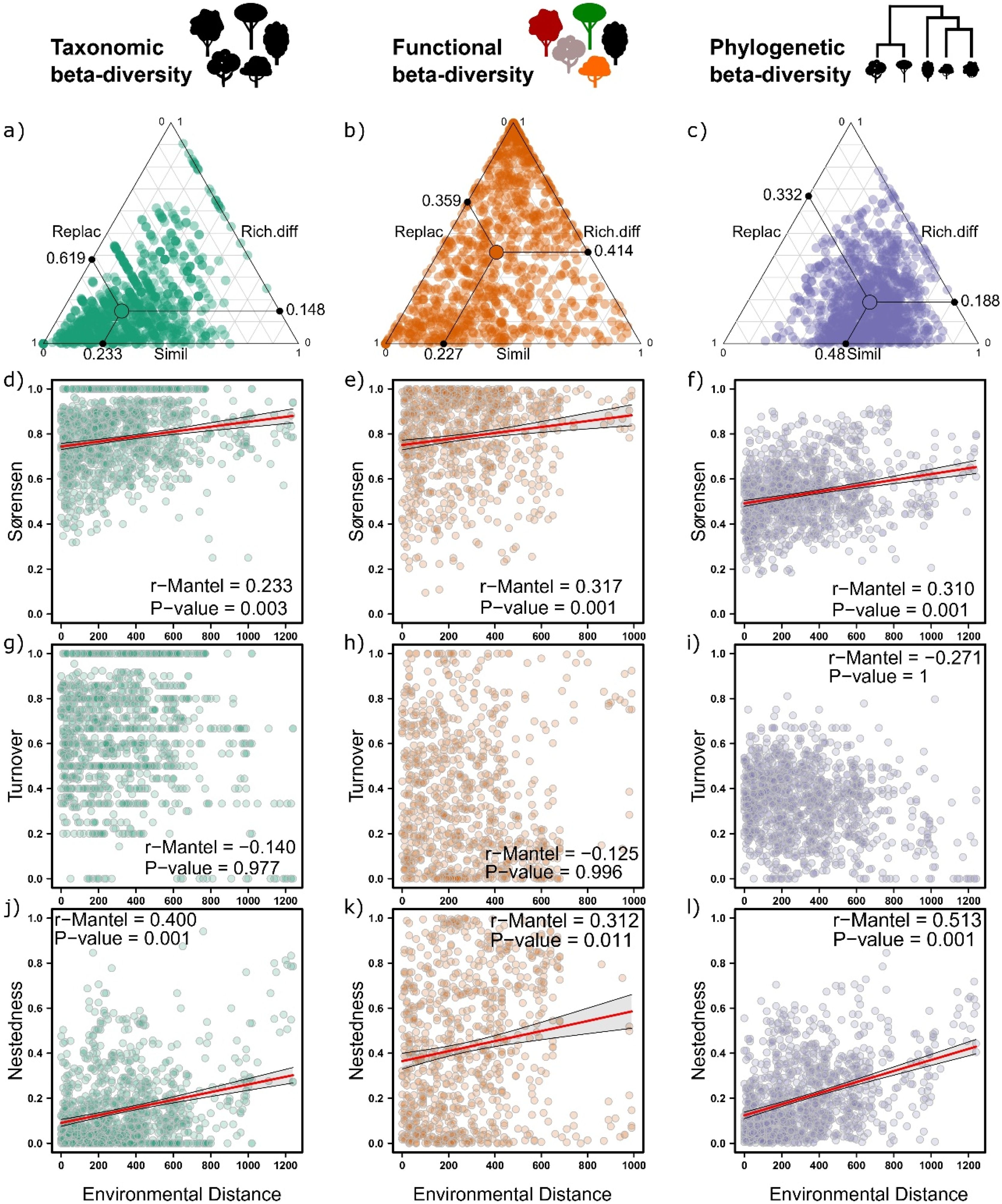
Relationship between biotic dissimilarity and environmental distance (açaí clump density) using incidence in relation to adult woody assemblages of an Amazonian estuarine forest. Total dissimilarity (β Sorensen) and its components (β Turnover and β Nestedness). Both determination coefficients (r) and significance (p) for each relationship are shown.
Information on plant traits is derived from the previous study in our focal sites (Freitas et al., 2021). Briefly, the functional trait values were obtained for 120 tree species by measuring five individuals per species. Trait measurement followed protocols available in the literature (Pérez-Harguindeguy et al., 2013). Among the nine attributes evaluated by the Freitas et al. (2021), we selected three that express conflicting demands in the allocation of resources present in the leaf economic spectrum: (i) specific leaf area (mm²/mg); (ii) leaf dry matter content (mg/g); (iii) leaf thickness (mm), which are expected to express a species-level gradient from conservative to acquisitive resource use strategies (Wright et al., 2004) Specific leaf area, leaf dry matter content and leaf thickness reflect adaptive conditions for plants relative to energy demand and water balance; these traits correlate with resource use strategies, and the trade-off between photosynthetic potential and nitrogen acquisition and herbivory (Wright et al., 2004; Díaz et al., 2016; Pérez-Harguindeguy et al., 2013). Particularly in the case of açaí intensification, it is expected a higher contribution of more acquisitive resource use strategies as palm density increases and, consequently, forest stands become more illuminated, in the expenses of conservative strategies (Freitas et al., 2021; Wright et al., 2004).
Scores of beta diversityFirst of all, a phylogenetic tree was obtained by estimating the continuous phylogenetic distance among the sampled species via Bayesian inference in a Markov chain Monte Carlo method (MCMC). This phylogenetic tree consisted of 206 species and 46 families (Fig. S2). The taxonomic, phylogenetic and functional beta diversity, and their components were based on the pairwise-site method. Before calculations, phylogenetic and functional information was adjusted to the taxonomic matrix. The Sorensen dissimilarity coefficient was used to calculate the total beta diversity, turnover and nestedness components (Socolar et al., 2016; Swenson et al., 2011). Beta diversity was calculated based on the presence of species at each site. It is important to mention that the taxonomic dimension was based on 47 sites and 203 species, the phylogenetic dimension covered 203 species across 47 sites, while the functional covered 43 sites and 106 species. Beta diversity calculations were carried out in R 4.0.1 software (R Core Team, 2021). Functions were used to calculate the taxonomic, phylogenetic and functional beta diversity pairwise matrix using the “betapart”, “beta.pair”, “phylo.beta.pair” and “func.beta.pair” packages respectively. All functions compute three distance matrices accounting for the (i) turnover (replacement-βsor), (ii) nestedness-resultant component (βsne), and (iii) total dissimilarity (i.e., the sum of both components - βsim). This approach, including the data analysis as follow, has been successfully applied elsewhere (Gómez‐Rodríguez and Baselga, 2018; Carvalho et al., 2020; Li et al., 2021).
Data analysisTo examine the açaí intensification effects on tree-assemblage beta diversity (distance-decay relationship), we adopted an environmental distance matrix based on açaí clump density per ha and correlated it with the beta diversity scores across the 47 sites. The Euclidean distance was used as the pairwise index in each site and a Pearson partial Mantel test with 9999 permutations was run in joint with a third spatial distance matrix. The partial Mantel statistic uses partial correlation conditioned on the third matrix, once the first matrix is permuted so that the correlation structure between the second and first matrices is kept constant (Legendre and Legendre, 2012). The environmental and spatial distance matrices and the Pearson partial Mantel test were performed in the “vegan” package, using “vegdist” and “mantel.partial” functions, respectively. To evaluate in which extent the sampling sites contribute to species nestedness along the açaí related gradient, we adopted a piecewise regression to test this relationship, where the contribution of each site (47 sites) to the nestedness was defined as the response variable, and the açaí management gradient was used as the explanatory factor. We used "bipartite" package, "nestedcontribution" function to calculate the individual nestedness contribution, and "segmented" packages to run the Piecewise regression, through "segmented" function. Finally, the contribution of particular species relative to beta diversity and tree assemblage organization along the açaí gradient were examined via threshold indicator species analyses (Baker and King, 2010).
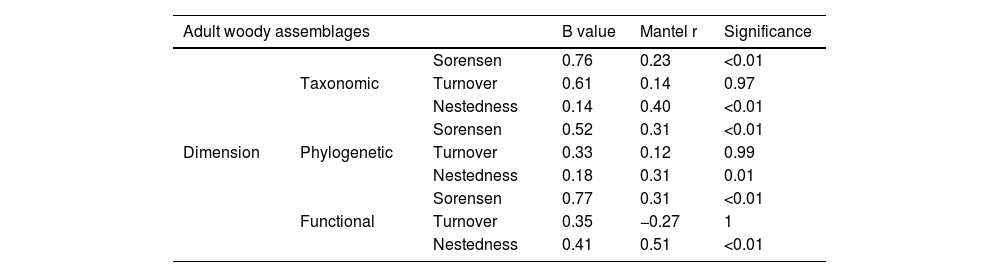
ResultsTree assemblage beta diversity was highly variable across forest stands considering all diversity dimensions, with dimensions/scores affected by both species turnover and nestedness (i.e., species loss), but the relative contribution of these diversity components varied according to the dimension. Both taxonomic and phylogenetic were more affected by turnover than nestedness (Table 1; Fig. 1a and c). In contrast, for functional beta diversity, nestedness was the component that most contributed to total beta diversity (Table 1; Fig. 1b).
Partial Mantel results (Pearson method) showing the relationship between woody plant assemblage dissimilarity (taxonomic, phylogenetic and functional) and environmental distance (açaí clump.ha−1) of an Amazonian estuarine forest, Brazil.
| Adult woody assemblages | B value | Mantel r | Significance | ||
|---|---|---|---|---|---|
| Dimension | Taxonomic | Sorensen | 0.76 | 0.23 | <0.01 |
| Turnover | 0.61 | 0.14 | 0.97 | ||
| Nestedness | 0.14 | 0.40 | <0.01 | ||
| Phylogenetic | Sorensen | 0.52 | 0.31 | <0.01 | |
| Turnover | 0.33 | 0.12 | 0.99 | ||
| Nestedness | 0.18 | 0.31 | 0.01 | ||
| Functional | Sorensen | 0.77 | 0.31 | <0.01 | |
| Turnover | 0.35 | −0.27 | 1 | ||
| Nestedness | 0.41 | 0.51 | <0.01 | ||
Açaí environmental dissimilarity (as a difference between each pair of forest stands relative to açaí clump density) correlated positively with total beta-diversity considering all diversity dimensions (Fig. 1d–f); i.e., the higher the difference in terms of clump density, more dissimilar were forest stands.However,changes in beta diversity associated with açaí environmental dissimilarity were not due to species turnover/replacement (Fig. 1g–i) but resulted from loss/gain of species (Fig. 1j–l). Moreover, forest stands with less than 400 açaí clump/ha−1 exhibited higher scores of species accumulation or beta diversity (Fig. 2). Collectively, these findings (Figs. 1 and 2) implied that as forest stands experience intensification, more dissimilar and species-impoverished they become (taxonomic, phylogenetically and functionally) as compared to those supporting natural densities (20–210 clumps per hectare) or low-intensity management (<400 clump/ha−1).
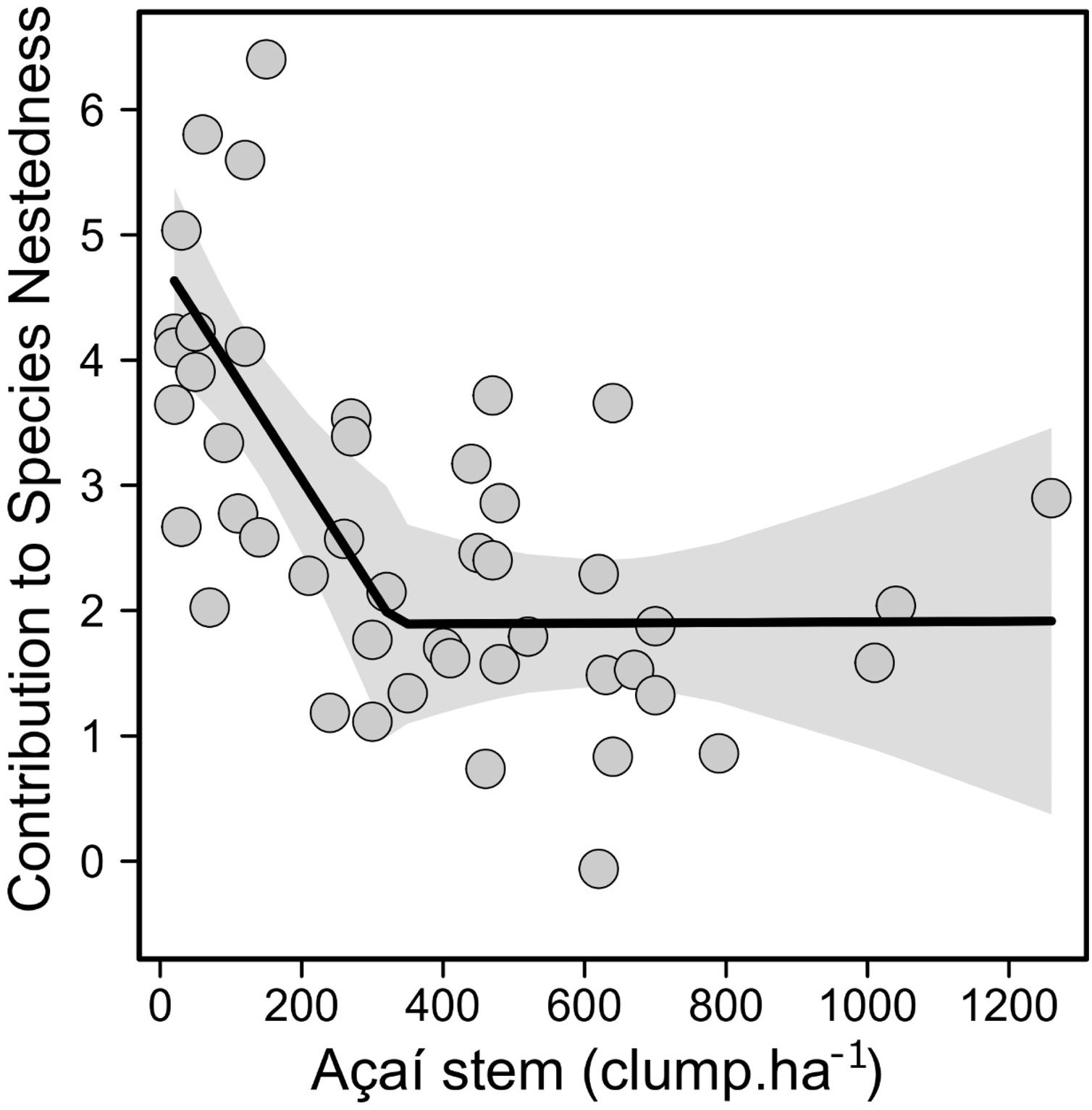
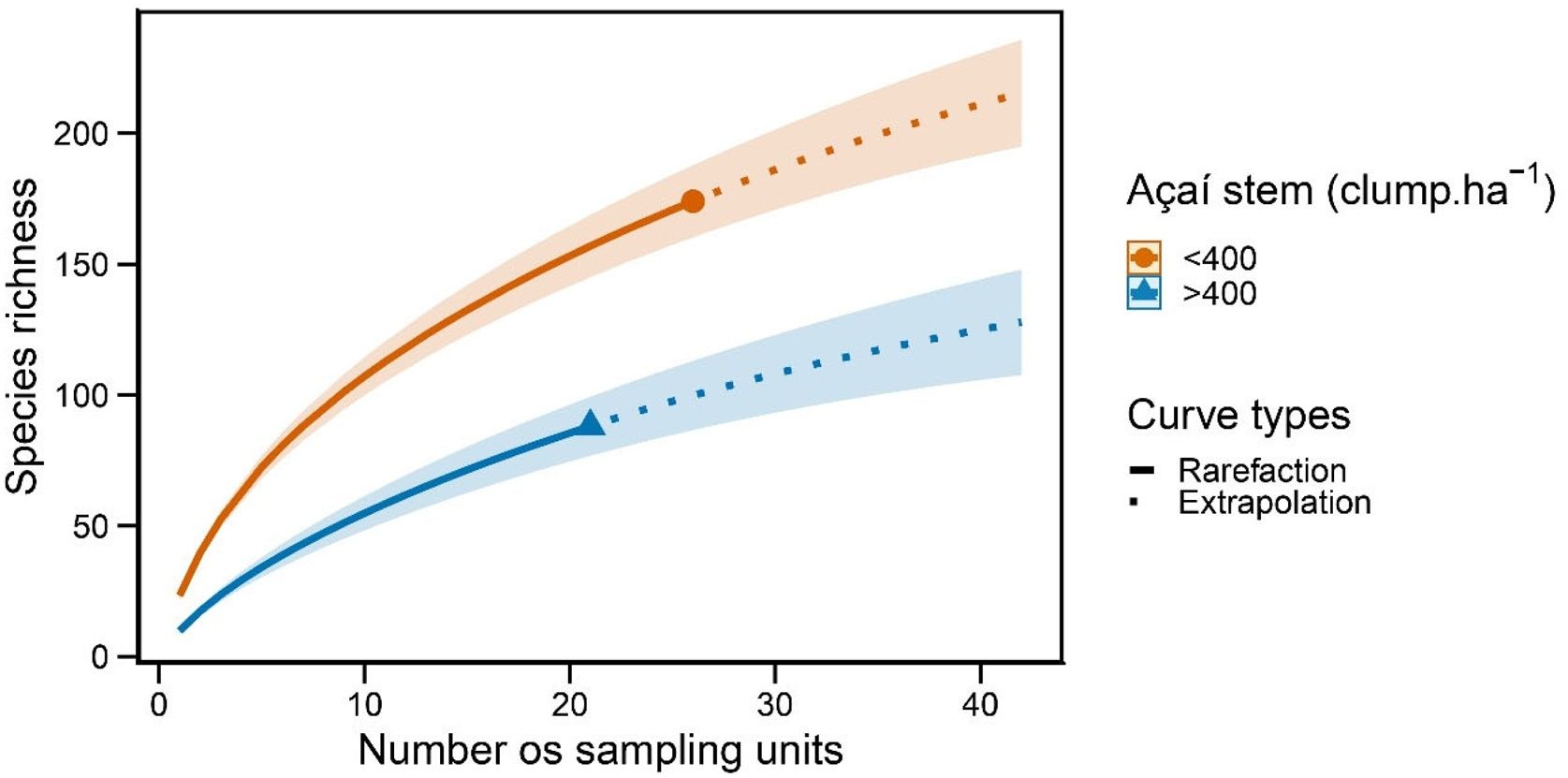
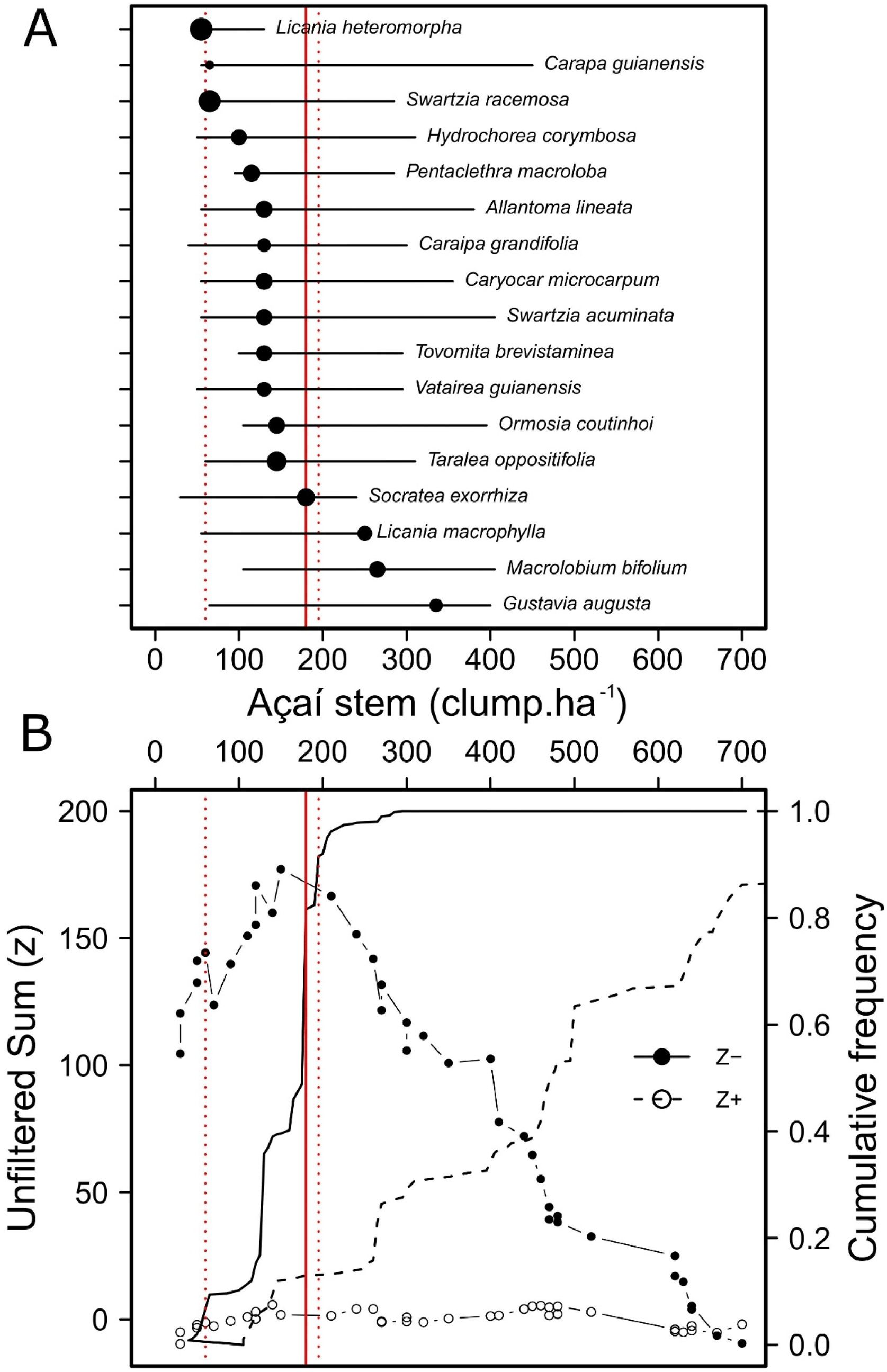
Accordingly, forest stands that most contributed to the total nestedness of the regional community were those with less açaí clumps and greater species richness (Fig. 3); i.e., probably because they contained the bulk of the estuarine woody flora. The piecewise regression model showed that 345 açaí clump.ha−1 is the threshold (breakpoint, Fig. 3) indicating the end of açaí clump density on nestedness (<345: t24 = −3.20 [CI: −0.0126, −0.00288], slope = −0.0077; >345: t23 = −0.0005 [CI: −0.0029, 0.0017], slope = −0.0005). Finally, it was possible to identify the tree species responding to changes on açaí clump density and then the impact on tree assemblage structure, including a reduction in beta diversity as açaí increments. While no species responded positively (increasing in abundance) to increments on açaí clump density, 17 species were indicated to negatively respond to increments on açaí (Fig. 4a). Moreover, the community-level threshold point was 180 clump/ha-1, ranging from 85 to 196 (0–95th percentile; Fig. 4b). It implied that (1) this species group was the first to be extirpated from forest stands as açaí clump density increased, and (2) after the 400-clumps threshold (i.e., the regulatory limit) almost half of the community was lost (>400 açaís clumps = 174 species; <400 açaís clumps = 91 species), with low turnover of species further (Fig. 4b). This set of sensitive species included several ecological and functional groups such as (1) emergent large-tree species (Pentacletra macroloba), canopy (Licania macrophylla) and understorey species (Tovomita bravistaminea), (2) hard (Taralea oppositifolia) and softwood species (Hydrocorea corymbosa).
Threshold Indicator Taxa ANalysis (TITAN) revealing the response of woody plant assemblages to variation in açaí clump density across 47 forest stands within an Amazonian estuarine forest in Brazil. (A) Indicator species significantly associated with increasing açaí clump density are represented by closed circles. Each circle denotes the estimated change point (threshold) in the abundance of a species along the açaí stem density gradient (clumps·ha−¹), while the horizontal lines represent the 5th and 95th percentiles obtained from 5,000 bootstrap replicates, indicating the uncertainty around each threshold estimate. (B) Sum(z) scores for negatively (z−, solid circles) and positively (z+, open circles) responding taxa plotted across the açaí stem gradient. These scores reflect the strength and consistency of species' responses at different points along the gradient. The cumulative frequency curves represent the proportion of taxa with consistent change points. The vertical solid red vertical line indicates the simulated community-level threshold — the point along the açaí density gradient at which the most substantial change in the composition of indicator taxa occurs. The vertical dotted red lines delimit the 95% confidence interval for this threshold, reflecting its statistical uncertainty. The figure highlights that community shifts were primarily driven by declines in the abundance of sensitive indicator species in response to increasing açaí dominance.
Our results suggest that the açaí intensification reduces the taxonomic, phylogenetic and functional beta diversity of the assemblages of the Amazon estuarine forest at regional scale. This sort of biodiversity loss associated with açaí intensification results from species loss along the intensification gradient. In other words, as açaí clump density increases, tree assemblages become more species-poor, with high-density forest stands capturing just a sub-sample of tree flora inhabiting those stands with low açaí density. Accordingly, at large spatial scales, the estuarine forest with açaí occurring through natural abundance will retain much more biodiversity than forest managed to artificially increment açaí clump density (i.e., reduced gamma diversity). Such a broad spatial-scale species loss affects tree species richness covering a wide range of ecological groups or life-history strategies. Furthermore, most of the species loss occurs up to the threshold of 400 clump.ha−1 (i.e., the regulation threshold), indicating that the species loss starts as soon as açaí fruit production moves from extractivism from natural population to management, first at forest-stand scale, but further at landscape and regional scale as intensification encroaches the estuarine forest. Finally, no tree species benefits from intensification at regional scale.
These findings largely extend our knowledge about the effects posed by the açaí intensification on the integrity of the estuarine forest, particularly in the case of tree assemblages (Freitas et al., 2015, 2021, Barros et al., 2023). It has been reported a decline in tree stem abundance achieving up to 84% at forest-stand scale by considering the whole gradient of açaí clump density (20–1260 clump.ha−1). This drastic change correlates with (1) a decline on tree species richness (up to 87%), (2) changes on functional diversity, (3) the persistence of a small set of exploited tree species, (4) the emergence of distinct taxonomic assemblages, and (5) a 50% reduction by forest basal area at landscape spatial scale; i.e., a set of forest stands into the same forest site or locality (Freitas et al., 2015, 2021). Not only tree assemblages (≥5 cm DBH) become impoverished at forest-stand scale, but also the understory woody plant assemblages consisting of shrubs, small trees, and saplings from canopy and emergent tree species as demonstrated by Barros et al. (2023). Briefly, açaí intensification implies the erosion of tree assemblages, at multiple spatial scales, by the elimination of tree species covering a wide spectrum of ecological groups with no species benefiting from intensification, although it has been proposed (but still not tested) that intensification result into more open (reduced leaf cover), illuminate and desiccated forest stands and thus might favor light-demanding species (see Carreño-Rocabado et al., 2016; Craine, 2009). Such a fast biotic impoverishment also involves the functional dimension considering a wide range of traits relative to leaf economic spectrum and the life history strategies of plants present in traits such as maximum plant height, stem specific density, specific leaf area, leaf dry matter content, leaf thickness, stomatal density, stomatal area and seed mass (Freitas et al., 2021; Barros et al., 2023).
In this context, our findings do not support potential trajectories experienced by disturbed biotas such as a small set of winning species replacing a diverse set of losing species (Tabarelli et al., 2012; Filgueiras et al., 2021). According to the winner-loser paradigm (Tabarelli et al., 2012), human disturbance is expected to facilitate the proliferation of disturbance-adapted species, including those from adjacent biotas able to immigrate and then take part of the community assembly (Kramer et al., 2023; Rolls et al., 2023). This common response to disturbance is one of the processes leading to biotic homogenization (Lôbo et al., 2011; McKinney and Lockwood, 1999), while the persistence of disturbance-sensitive species via low frequency across disturbed patches reduces it (Lazzaro et al., 2015; Solar et al., 2015). It is true that some species tend to be intentionally spared across açaí high-density forest stands (e.g., Pterocarpus santalinoides and Mauritia flexuosa), while a more illuminated habitat shall favor some pioneer or light demanding flora. However, constant forest thinning eliminates these possibilities does not permitting disturbance-adapted tree species to thrive across high-density açaí forest stands and thus cause biotic homogenization as already documented across disturbed tropical forests (Lôbo et al., 2011; McKinney and Lockwood, 1999; Tabarelli et al., 2012).
Moving further into the underlying mechanisms, reduced species richness at forest stand level, i.e., reduced alpha-diversity as already reported (Barros et al., 2023; Freitas et al., 2015, 2021) does not necessarily result in decreasing beta diversity considering all community-level dimension as documented here. In this context, an extensive list of mechanisms has already been proposed to explain the emergence of low-density and impoverished woody plant assemblages in response to açaí intensification at forest-stand scale although empirical evidence remains scarce or completely absent. We shall mention (1) limited seed rain and low-density impoverished soil seed bank, (2) changes on microclimate conditions disfavoring the old-growth shade-tolerant flora, (3) seedling suppression by açaí leaf-litter, and (4) elimination of undesired species to give extra space for açaí recruitment and growth (Barros et al., 2023; Freitas et al., 2015, 2021; Silva et al., 2023). Collectively, these drivers make it reasonable to propose that high-density açaí forest stand, in addition to low-density and tree species-poor, capture just a subset of the regional community. Namely, the set of useful species which are intentionally spared during thinning/coppicing operations (every 2–3 years), but also those able to achieve (i.e., allochthonous seed dispersal) and recruit across few patches by tolerating illuminated habitats and perhaps able to resprout in response to frequent coppicing while forest stands are not completely transformed into açaí monospecific stands; i.e., the elimination of undesired species plus environmental filtering reorganizing patterns of beta diversity via species loss causing functional, phylogenetic and taxonomic impoverishment at multiple spatial scales.
Similar to other NTFPs, açaí is rapidly moving from simple extractivism for management, and industrial production. Demand for açaí fruits will continue to increase as açaí-based products gain new international markets (Silveira et al., 2023), leading to a recent proliferation of fruit-processing plants established in the estuarine region. In 2022, the Pará state alone produced 164,900 tons of raw fruits (IBGE, 2022), most of it from the estuarine forest as in the previous years (Tavares et al., 2020). In this context, direct fruit acquisition from the traditional riverine families by corporations is probably a driver for intensification by providing technical and economic assistance, including better prices as compared to those offered by traditional merchants (Antunes et al., 2021; Tavares et al., 2020). This is additional evidence that açaí fruit production in the eastern Amazon has already crossed the limit from the extractivism phase and entered into the industrial production via managed “natural” populations in the estuarine forest. In other words, the açaí forest habitat that is threatened or experiencing degradation rather than the açaí populations.
In this context, it is worth mentioning that the Amazon estuarine forest supports a diverse flora, including a large number of tree species typical form undisturbed forests such as those from the old-growth families Sapatoceae, Lecythidaceae, Chrysobalanaceae, and Anonnaceae (Freitas et al., 2021; Barros et al., 2023). Moreover, the ecosystem services provided by the estuarine forest (in addition to supporting a traditional human culture) are still unknown. The estuarine forest provides some irreplaceable ecosystem services of local and global relevance, such as the production of biomass and the incorporation of an immense amount of litter material (seeds, fruits, leaves, branches etc.) into the aquatic systems via the tide regime. Please note that the Amazon estuary is one of the most productive fishery regions globally (Sodré et al., 2011). It is true the açaí fruit production/harvesting has (1) enhanced the livelihood standards by riverine populations via increasing incomes/revenue, and (2) developed local/regional economies by supporting entire processing/commercialization chains from raw fruits to food/supplement, cosmetic and health items (Antunes et al., 2021; Laurindo et al., 2023; Magrach and Sanz, 2020; Veloz, 2020; Weinstein and Moegenburg, 2004), while the target species is not thereat by overexploitation. Accordingly, açaí fruit production as other NTFPs persists as an opportunity to reconcile forest integrity and a better life for traditional, in many cases vulnerable populations currently responsible for a considerable proportion of the remaining tropical forests in their territories.
However, açaí intensification in the expense of tree assemblages (i.e., understory, canopy and emergent assemblages) and forest physical structure leading to species loss and reduced beta diversity at regional level clearly represents forest degradation (sensu Lapola et al., 2023). This impoverishment depends on which extension managed, high-density açaí stands replace the forest patches supporting açaí natural densities, as indicated by Fig. 2. Such a transition by the Amazonian “black gold” has both theoretical and applied implications, perhaps lessons, particularly by considering the current claim for an Amazonian development based on forest bioeconomy based on the exploitation of the sociobiodiversity through sustainable chains (Costa et al., 2021; Delgado et al., 2023; Laurindo et al., 2023; Veloz, 2020). First, it calls attention to how fragile and ephemeral the sustainable production of NTFPs can be as markets pose a demand beyond the threshold represented by the yields supported by natural populations, while it offers additional revenue coming from industrial productions. Second, açaí regulation limiting intensification, is highly appreciated and crucial to avoid a massive conversion of native forests into açaí monoculture. However, intensification level (i.e., not only a single rule, but one defining a maximum clump density is mandatory), must be a compromise between economic viability, forest integrity, ecosystem services and biodiversity persistence, but also forest resilience to climate changes. In other words, both the açaí palm species and the managed, largely impoverished açaí-dominated forests can be sensitive to droughts and decreasing levels of precipitation as predicted for the region (see Sakschewski et al., 2016; Levine et al., 2016), undermining a socioecological system based on fruit production (Evangelista-Vale et al., 2021). Considering all these implications and potential scenarios, a research program is required addressing both the familiar/traditional production, but also the commercial/industrial enterprises, i.e., an emerging trend in response to the proliferation of açaí-processing industrial plants in the region.
Finally, the açaí opportunities/threats should be considered a central component into a regional strategy to promote the sustainable development of the estuarine region, probably demanding the establishment of protected areas and the restoration of severally degraded forests with a focus on already rare tree species and multiple ecosystem services. They shall guarantee the long-term persistence of the last tracts of the old-growth forest in the region as a permanent control for the açaí experiment/case, which test and shall offer globally-relevant lessons relative to NTFPs as tool for the sustainable use of tropical forests (and its corollary relative to socioeconomic development) rather than another driver of forest degradation in addition to habitat loss, edge-effects, logging and droughts associated with climate changes (see Lapola et al., 2023; Flores et al., 2024).
ORCIDMadson Antonio Benjamin Freitas: 0000-0002-6451-9405
Arleu Barbosa Viana-Junior: 0000-0002-9964-9875
Maria Fabiola Barros: 0000-0003-0284-8438
Ima Célia Guimarães Vieira: 0000-0003-1233-318X
Elâine Maria dos Santos Ribeiro: 0000-000 2-3 632-1004
CRediT authorship contribution statementMadson Antonio Benjamin Freitas: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing – original draft; Writing – review and editing.
Arleu Barbosa Viana-Junior: Data curation; Formal analysis; Investigation; Writing – review and editing.
Maria Fabíola Barros: Conceptualization; Investigation; Methodology; Writing – review and editing.
Leonardo Magalhães: Conceptualization; Investigation; Methodology; Writing – review and editing.
Elâine Maria dos Santos Ribeiro: Data curation; Formal analysis; Investigation.
Ima Célia Guimarães Vieira: Conceptualization; Investigation; Writing – review and editing.
Marcelo Tabarelli: Conceptualization; Investigation; Methodology; Writing – review and editing.
This study was partially funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Código Financeiro 001 and by two INCT projects (CNPq grant 574008/2008 and 406516/2022-7). MABF was supported by a PDJ-CNPq scholarship (168392/2022-4 within the scope of call 008/2022) and Fapespa/CNPq (grant 2023/158408). We also thank the Graduate Program in Plant Biology of Universidade Federal de Pernambuco, Camila Martins and Júlio Alves for the help with analyses. ICGV and MT received support from CNPq (Proc. 350182/2022-1 and 314215/2021-2). We appreciate the criticism offered by the three reviewers and the Editor-in-Chief.