Indigenous peoples and local communities (IPLC) manage over half of the world’s landscapes, and this management involves landscape transformations associated with their sociocultures. Although anthropologists have shown that IPLC sociocultures influence management, and historical-ecological studies have shown that this management influences environments, how interactions between IPLC sociocultures and environments influence landscape transformations is less clear. Here we use a historical-ecological approach and a cultural niche construction perspective to present an IPLC landscape transformation framework that identifies and integrates sociocultural and environmental elements. Our framework shows that IPLC’ landscape transformations occur through cultural niche construction and are influenced by historical events. IPLC sociocultures influence ecological processes and patterns through interactions that create sociocultural and ecological inheritances. These inheritances involve IPLC worldviews and associated norms, practices and knowledge which influence ecological processes that, in turn, engender ecological patterns. On the other hand, ecological processes and patterns influence IPLC sociocultures as they are perceived and processed according to local worldviews, so generating sociocultural–environmental feedbacks. To exemplify our framework, we present cases of cultural niche construction by Amazonian IPLC that show how interactions between sociocultures and environments influence landscape transformations. We argue that understanding how IPLC sociocultures have interacted with environments can help scientists, conservation practitioners and policymakers to combine scientific knowledge production, biodiversity protection and IPLC’ well-being.
Indigenous peoples and local communities (IPLC)1 occupy over 50% of the world’s landscapes (Pearce, 2016). IPLC have sustained their livelihoods (Reyes-García, 2015), protected carbon stocks (Rights and Resources Initiative, 2018), contributed to ecological restoration (Reyes-García et al., 2019), and conserved biodiversity (Porter-Bolland et al., 2012) while transforming their landscapes. Yet, governments generally exclude IPLC from decision-making that affects them and impose public policies that disregard IPLC lifestyles, causing damage to local health, food sovereignty and biodiversity (Apina et al., 2017; MacInnes et al., 2017; Andrianto et al., 2019). The ways IPLC transform their landscapes in South America (Viveiros de Castro, 2012; Fernández-Llamazares et al., 2020), North America (Levi-Strauss, 1983; Blaser, 2016), Africa (Forde, 1999; Murove, 2004), Europe (Elbakidze and Angelstam, 2007; Babai and Molnár, 2014), Asia (Luo et al., 2009; Anthwal et al., 2010) and Oceania (Memmott and Long, 2002; Knudsen, 2004) are guided by their sociocultures (e.g., worldviews, norms and knowledge). These transformations, in turn, have influenced ecological processes and patterns around the world (Boivin et al., 2016; Levis et al., 2017; Roberts et al., 2017). However, how IPLC sociocultures interact with ecological processes and patterns is less clear. Amid biodiversity losses (Johnson et al., 2017), ethnocide/epistemicide (Hall and Tandon, 2017) and climate crisis (Ripple et al., 2019), this understanding is important to combine scientific knowledge production, biodiversity conservation and IPLC’ well-being.
IPLC' landscape transformations depend upon local sociocultures and environments (Beltrán, 2000; Balée, 2006; Berkes, 2017). Thus, we expect that different sociocultures interact with ecological processes and patterns differently. Since historical events (e.g., wars, colonization and migrations) influence these interactions (Balée, 2009; Crumley, 2017; Walters et al., 2019), IPLC’ landscape transformations can be better understood through the historical ecology research program. Historical ecology proposes that historical events have influenced landscape transformations by influencing sociocultures, so that landscapes are legacies in constant transformation that represent diverse space-time encounters of places, humans and non-humans whose histories are imprinted in the environment (Balée, 1998, 2006).
IPLC’ landscape transformations also have been understood through the cultural niche construction perspective (Boivin et al., 2016; Albuquerque et al., 2018). Niche construction theory proposes that organisms modify environments to make available conditions/resources not previously present, thus changing local selective pressures that act on the organisms (Odling-Smee et al., 2013). Cultural niche construction proposes that humans modify environments using information that is socioculturally learned, and that future generations inherit these modified environments and are influenced by them. Cultural niche construction involves selective pressures that act not only on human biology, but also on human culture (Odling-Smee and Laland, 2011). While historical ecology emphasizes the role of historical events in sociocultural–environmental relationships, cultural niche construction emphasizes the role of sociocultural transmission in these relationships. Therefore, the cultural niche construction perspective can contribute to historical ecology to clarify how sociocultures interact with ecological processes and patterns.
Here, we present a framework for identifying and integrating sociocultural and environmental elements of IPLC’ landscape transformations. Our framework expands existing social-ecological approaches (e.g., Berkes et al., 2000; Ostrom, 2009; Díaz et al., 2015; Levis, 2018; Schlüter et al., 2019) by showing how IPLC sociocultures interact with ecological processes and patterns through cultural niche construction. We exemplify our framework by presenting cases of IPLC cultural niche construction in Amazonia, a region that harbors the greatest biodiversity of all tropical rainforests (Hansen et al., 2013) and thousands of IPLC (Adams et al., 2008; Ricardo and Ricardo, 2017). Finally, we highlight how our framework can be used by scientists, conservation practitioners and policymakers in order to generate scientific knowledge, biodiversity conservation and IPLC’ well-being.
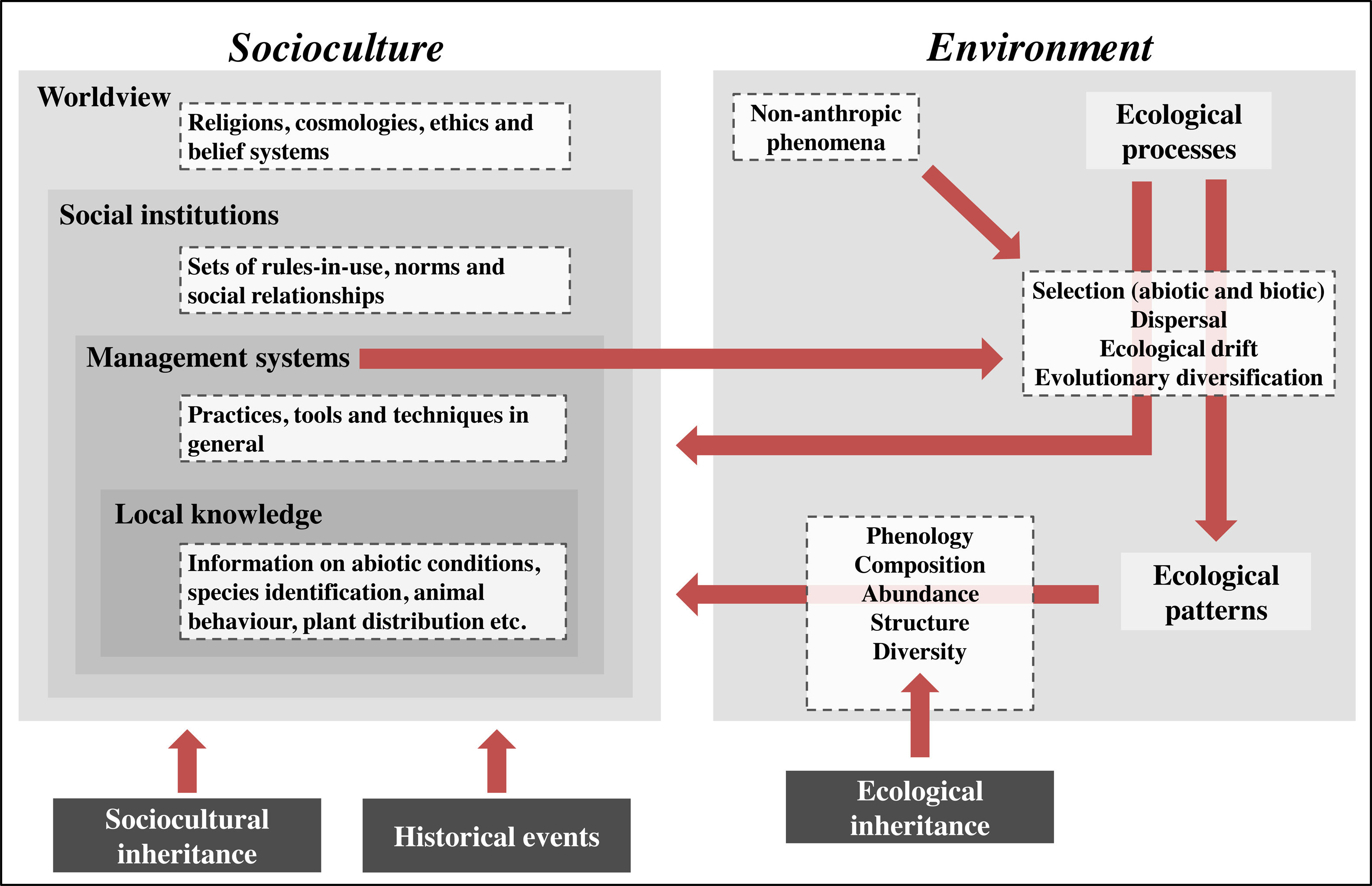
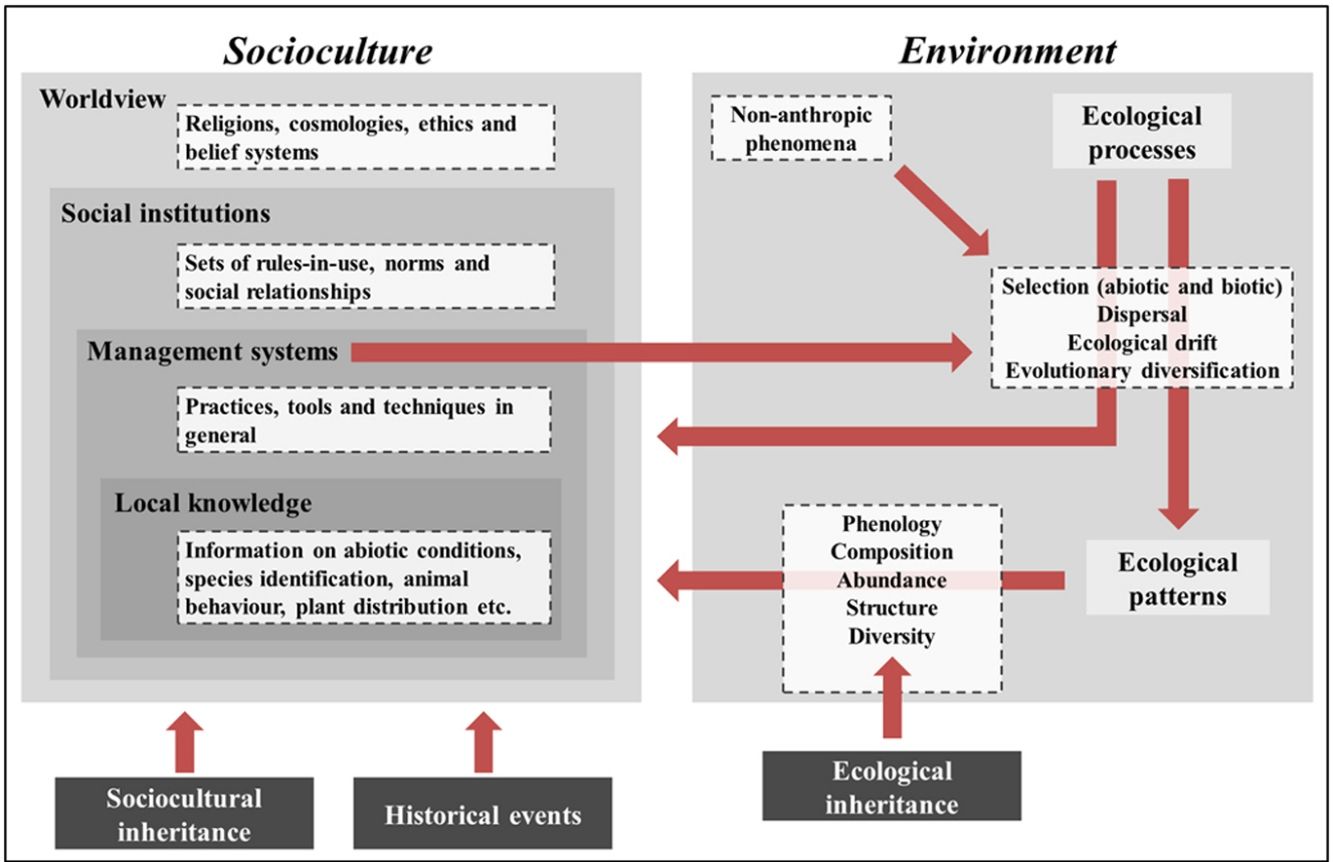
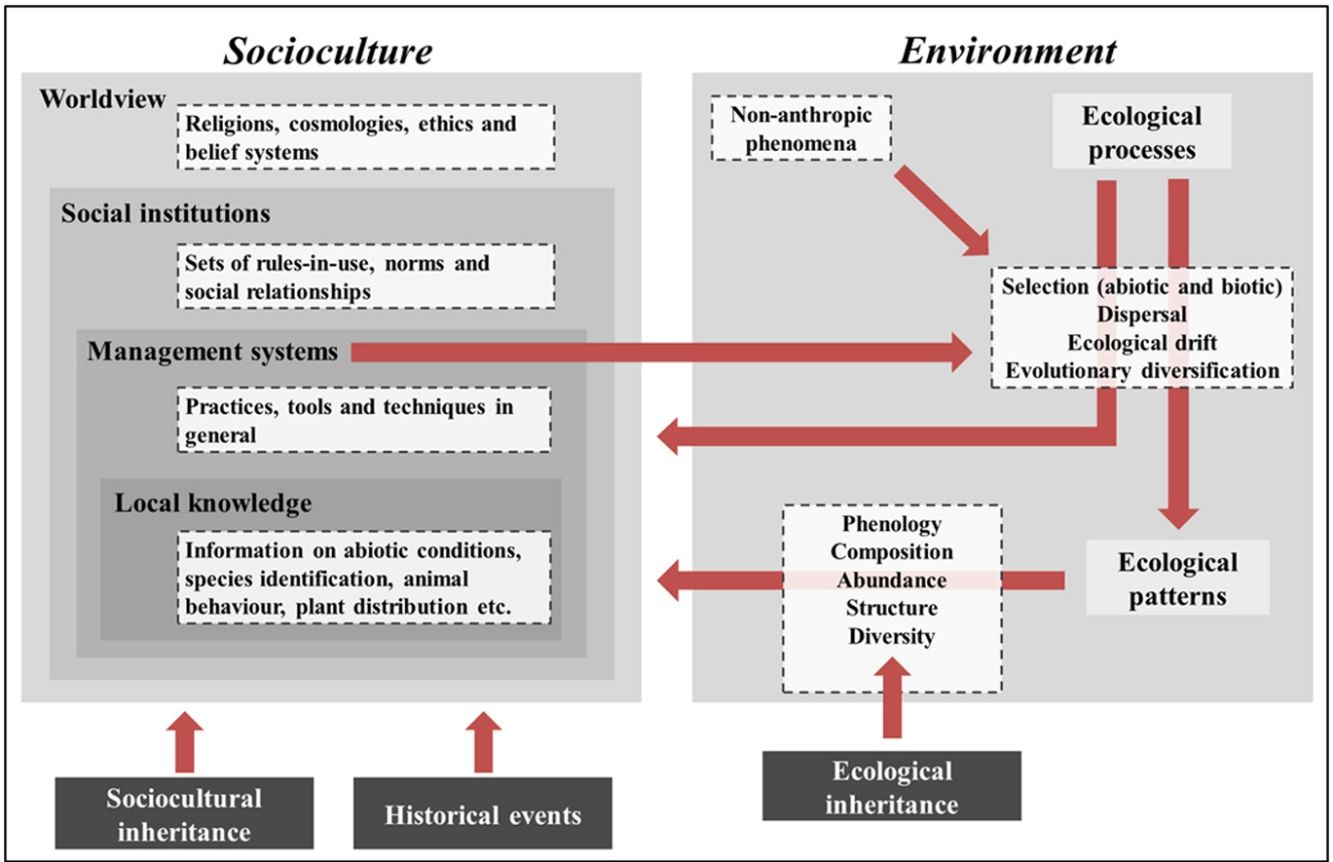
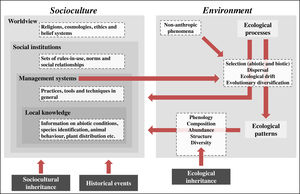
IPLC landscape transformation frameworkOur framework identifies sociocultural and environmental elements involved in IPLC’ landscape transformations through anthropological and ecological concepts, and integrates them using a historical-ecological approach and a cultural niche construction perspective (Fig. 1). In our framework, socioculture includes the four components of traditional ecological knowledge (TEK)2 (Berkes et al., 2000): worldview, social institutions, management systems and local knowledge (see Table 1 for definitions). Worldviews vary among IPLC (Coscieme et al., 2020), from Native American societies whose cosmologies consider that non-humans have human-like cultures (Descola, 2005), to African societies that believe in spiritual powers emanating from ancestral spirits (Taringa, 2006), to Bangladeshian societies that comprehend their environments according to Christian doctrine (Rahmatullah et al., 2010). Social institutions are guided by worldviews and include hunting norms, such as in Native Canadian societies (Blaser, 2016), territorial divisions, such as in Amazonian societies (Wright, 1998), and food taboos, such as in Fijian societies (Henrich and Henrich, 2010). Management systems obey social institutions and range from Andean societies with irrigation systems (Trawick, 2001), to Amazonian societies practicing swidden-fallow cultivation (Denevan, 2001), to Mongolian societies managing their rangelands (Fernández-Giménez, 2000). Local knowledge underlies management and includes knowledge of animals, plants etc. Folk taxonomy of Tanzanian societies (Tibuhwa, 2012), animal behavior knowledge of Spanish pastoralists (Fernández-Giménez and Estaque, 2012) and forest succession classification of Indonesian societies (Poffenberger and McGean, 1993) are examples of local knowledge.
IPLC landscape transformation framework for identifying and integrating sociocultural and environmental components. IPLC’ landscape transformations occur through cultural niche construction and involve interactions among sociocultures and environments. Environment includes two components: ecological processes and ecological patterns. Pattern elements, such as species composition and abundance, can be generated through four ecological processes, which, in turn, are influenced by non-anthropic phenomena (e.g., windstorms, lightning and animal behaviors) and human management systems. Socioculture includes four components: worldview, social institutions, management systems and local knowledge. Management systems include human behaviors that transform landscapes via environmental manipulation that acts on ecological processes. Such management encompasses local knowledge and respects social institutions, which, in turn, are grounded in a local worldview. At the same time that socioculture transforms landscapes via environmental management, environment influences socioculture, either through ecological processes or ecological patterns, since both of them can influence all sociocultural components. These reciprocal relationships between socioculture and environment occur from generation to generation through cultural niche construction. For example, ecological patterns modified by one generation will be bequeathed by the next generation, so they represent an ecological inheritance. In addition, through orality, imitation and observation, the ways one generation thinks and acts (i.e., their worldview, social institutions, management systems and local knowledge) are transmitted to the next generation, so there is sociocultural inheritance across generations. Finally, historical events, by influencing socioculture, influence the way such reciprocal relationships between socioculture and environment occur in space-time, so that IPLC’ landscape transformations can best be understood by considering the role of history in shaping local sociocultures.
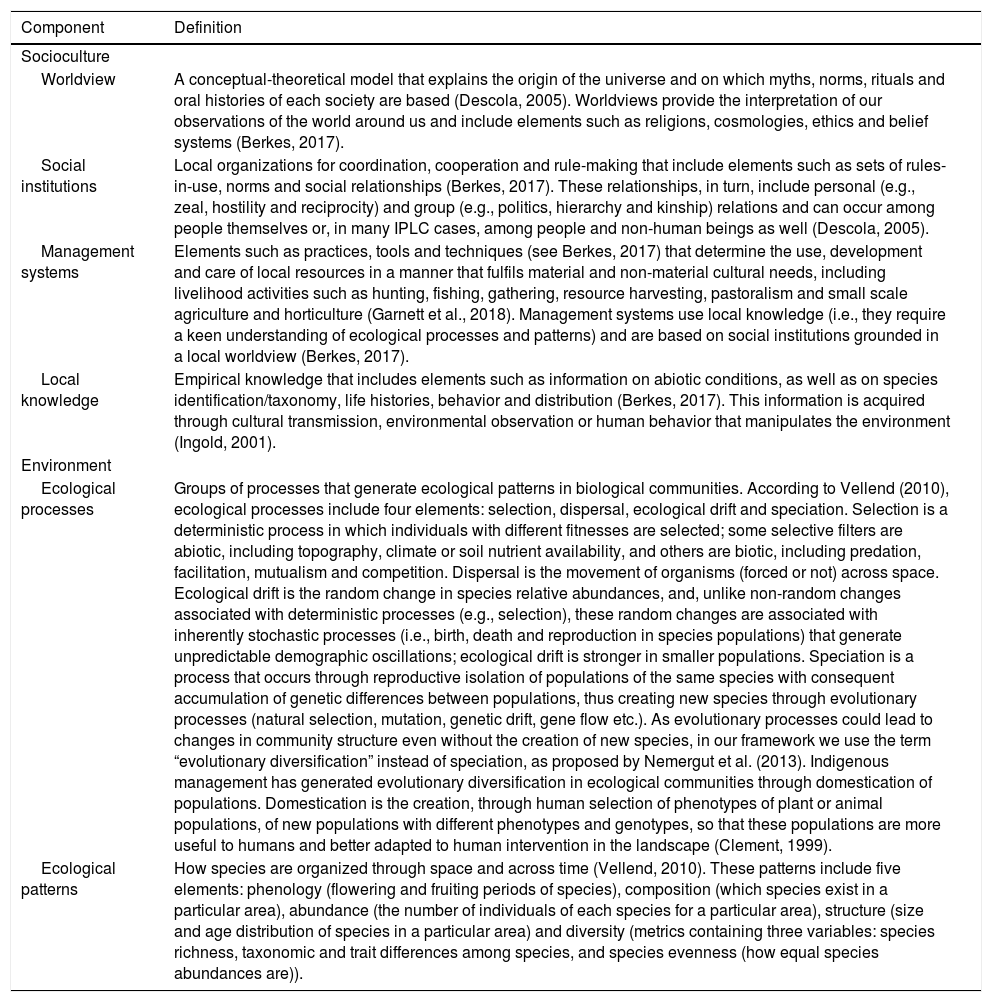
Definitions of sociocultural and environmental components.
| Component | Definition |
|---|---|
| Socioculture | |
| Worldview | A conceptual-theoretical model that explains the origin of the universe and on which myths, norms, rituals and oral histories of each society are based (Descola, 2005). Worldviews provide the interpretation of our observations of the world around us and include elements such as religions, cosmologies, ethics and belief systems (Berkes, 2017). |
| Social institutions | Local organizations for coordination, cooperation and rule-making that include elements such as sets of rules-in-use, norms and social relationships (Berkes, 2017). These relationships, in turn, include personal (e.g., zeal, hostility and reciprocity) and group (e.g., politics, hierarchy and kinship) relations and can occur among people themselves or, in many IPLC cases, among people and non-human beings as well (Descola, 2005). |
| Management systems | Elements such as practices, tools and techniques (see Berkes, 2017) that determine the use, development and care of local resources in a manner that fulfils material and non-material cultural needs, including livelihood activities such as hunting, fishing, gathering, resource harvesting, pastoralism and small scale agriculture and horticulture (Garnett et al., 2018). Management systems use local knowledge (i.e., they require a keen understanding of ecological processes and patterns) and are based on social institutions grounded in a local worldview (Berkes, 2017). |
| Local knowledge | Empirical knowledge that includes elements such as information on abiotic conditions, as well as on species identification/taxonomy, life histories, behavior and distribution (Berkes, 2017). This information is acquired through cultural transmission, environmental observation or human behavior that manipulates the environment (Ingold, 2001). |
| Environment | |
| Ecological processes | Groups of processes that generate ecological patterns in biological communities. According to Vellend (2010), ecological processes include four elements: selection, dispersal, ecological drift and speciation. Selection is a deterministic process in which individuals with different fitnesses are selected; some selective filters are abiotic, including topography, climate or soil nutrient availability, and others are biotic, including predation, facilitation, mutualism and competition. Dispersal is the movement of organisms (forced or not) across space. Ecological drift is the random change in species relative abundances, and, unlike non-random changes associated with deterministic processes (e.g., selection), these random changes are associated with inherently stochastic processes (i.e., birth, death and reproduction in species populations) that generate unpredictable demographic oscillations; ecological drift is stronger in smaller populations. Speciation is a process that occurs through reproductive isolation of populations of the same species with consequent accumulation of genetic differences between populations, thus creating new species through evolutionary processes (natural selection, mutation, genetic drift, gene flow etc.). As evolutionary processes could lead to changes in community structure even without the creation of new species, in our framework we use the term “evolutionary diversification” instead of speciation, as proposed by Nemergut et al. (2013). Indigenous management has generated evolutionary diversification in ecological communities through domestication of populations. Domestication is the creation, through human selection of phenotypes of plant or animal populations, of new populations with different phenotypes and genotypes, so that these populations are more useful to humans and better adapted to human intervention in the landscape (Clement, 1999). |
| Ecological patterns | How species are organized through space and across time (Vellend, 2010). These patterns include five elements: phenology (flowering and fruiting periods of species), composition (which species exist in a particular area), abundance (the number of individuals of each species for a particular area), structure (size and age distribution of species in a particular area) and diversity (metrics containing three variables: species richness, taxonomic and trait differences among species, and species evenness (how equal species abundances are)). |
Environment in our framework includes two components: ecological processes and patterns (Watt, 1947). Ecological processes are triggered by non-anthropic (animals, plants, wind etc.) or anthropic (management practices) phenomena and include four groups of processes, sensu Vellend (2010): selection, dispersal, ecological drift and evolutionary diversification (Table 1). Selection encompasses abiotic conditions, such as soil nutrient availability, temperature and precipitation (ter Steege et al., 2006), and biotic interactions, such as facilitation, predation and competition (Johnson et al., 2012). Management practices can act on biotic interactions or directly influence abiotic conditions. For example, consuming seeds and eliminating non-useful plants when opening swiddens (Levis, 2018) represent predation and competition, respectively, and fertilizing soils increases soil nutrient availability (Lehmann et al., 2003). Dispersal involves abiotic forces, such as wind and river movements (Umaña et al., 2011), animal behaviors, such as seed-defecation (Willson and Traveset, 2000), and management practices, such as transportation of plants and animals (Boivin et al., 2016). Ecological drift can be influenced by non-anthropic phenomena (e.g., hurricanes and windstorms) and management practices (e.g., clearings in forests/rangelands and exotic species introduction), both of which can reduce population sizes (Vellend, 2010; Gilbert and Levine, 2017). Evolutionary diversification results from evolutionary processes that change population genetic structure. IPLC have caused evolutionary diversification for thousands of years. For example, management practices (e.g., selecting individuals with desirable phenotypical traits) created hundreds of tree/crop varieties worldwide, including Brazil Nut (Bertholletia excelsa) and Breadfruit (Artocarpus altilis) (Meyer et al., 2012), as well as caused evolutionary changes in animals, such as bighorn sheep (Ovis canadensis) and pink salmon (Oncorhynchus gorbuscha) (Sullivan et al., 2017).
By influencing ecological processes, IPLC management indirectly affects ecological patterns, such as species phenology, composition, abundance, structure and diversity (Table 1). For example, in the southwestern United States, shrublands are burned to induce resprouting and flowering (Kimmerer and Lake, 2001); in Brazilian Amazonia, soil management influences soil bacterial community composition (Taketani et al., 2013); in India, fishing in wetlands increases bird species abundances (Aarif et al., 2017); in Canada, intertidal resource management modified forest structure (Trant et al., 2016); in Africa, savannah management increased faunal diversity (Marshall et al., 2018). Worldwide, most ecological patterns are niches culturally constructed by IPLC and represent ecological inheritances (see below) for current/future generations (Clement et al., 2020).
Our framework shows that management systems influence ecological processes that, in turn, engender ecological patterns, and both processes and patterns influence sociocultural components. These sociocultural–environmental feedbacks occur from generation to generation and involve sociocultural and ecological inheritances (Odling-Smee and Laland, 2011). If one generation thinks and acts in a certain way, the next generation will think and act in a similar way, except for some losses and innovations (sociocultural inheritance). Likewise, if people in one generation modify their environments, the next generation will inherit these modified environments, be influenced by them, and modify them again (ecological inheritance).
In our framework, sociocultural–environmental feedbacks are contingent upon history as historical events cause changes in sociocultures influencing their interactions with environments. In Tanzania, local policies forced changes in Maasai management systems. Hence, Maasai men usurped women's roles as traders of livestock products and started selling them, which changed local management and gender relations by dispossessing women’s rights over livestock products (Hodgson, 1999). In India, incorporation of Hinduism into traditional community beliefs caused changes in local worldviews, so that traditional management based on sacred tree groves has been replaced by the construction of temples within the forest (Chandran and Hughes, 1997). Worldwide, changes in IPLC’ religions because of colonialism, Jesuit missions or cultural transformation have resulted in changes in all sociocultural components (Brock, 2005).
Cultural niche construction by Amazonian IPLCWe present Amazonian IPLC examples that show how sociocultural–environmental interactions have influenced landscape transformations. The examples do not show all possible interactions among sociocultural and environmental elements, since there are no Amazonian studies that encompass all these interactions. Rather, they identify relations among some sociocultural and environmental elements to show how these elements can influence others. Below, elements are in italics and their components are in parentheses.
Sociocultural influences on environmentThe examples below illustrate sociocultural inheritances and show worldviews grounding social institutions that influence management systems, which, in turn, are based on local knowledge. They also show management systems influencing ecological processes that, in turn, engender ecological patterns, thus creating ecological inheritances.
In northwestern Amazonia, according to a Walipere-Dakenai (a Baniwa group) myth (worldview), the demiurge Ñapirikoli made their territory close to the Pamaale and Waraná streams (Wright, 1999). Animals were owners of edible fruit trees (e.g., Poraqueiba sericea and Tapirira guianensis), but Ñapirikoli stole these trees and provided the Walipere-Dakenai with information about tree distribution (local knowledge) (J. Franco-Moraes, personal observation, October-2015). This myth grounds a rules-in-use (social institution) that determines that the Pamaale-Waraná stream region only can be used (with few exceptions) by the Walipere-Dakenai (Wright, 1998). In this region, Walipere-Dakenai management practices (management system), such as unintentionally discarding seeds along trails and intentionally transplanting seedlings to gardens, which are dispersal and selection respectively (ecological processes), have influenced composition and increased the abundance (ecological patterns) of trees bequeathed by Ñapirikoli for centuries (Franco-Moraes et al., 2019). Moreover, local management caused evolutionary diversification (ecological process) of Humiria balsamifera, and a possible new variety of the species emerged and modified tree composition (ecological pattern) of the Pamaale-Waraná stream region (Abraão et al., 2010).
In the Xingu region, according to the Kalapalo cosmology (worldview), H. balsamifera is the chief of all trees. Their trunks are used to symbolize the body of dead Kalapalo chiefs during a ritual, where a norm (social institution) dictates that only medium-sized H. balsamifera trees can be cut (Guerreiro, 2011). The Kalapalo use information about species characteristics (local knowledge) in their technique (management system) of choosing/cutting medium-sized trees. Hence, this technique generates selection (ecological process) by not allowing H. balsamifera individuals to reach old age. This selection can decrease intra-specific asymmetric competition (i.e., greater resources use by old trees) and move competition towards an inter-specific asymmetric level (Freckleton and Watkinson, 2001). Since H. balsamifera is a large tree and a good inter-specific competitor (Franco-Moraes et al., 2019), by favoring inter-specific asymmetric level, Kalapalo selection can promote H. balsamifera abundance (ecological pattern). In fact, H. balsamifera occurs as dense populations in Kalapalo landscapes (Guerreiro, 2011).
In northwestern Amazonia, the Tukano cosmology (worldview) considers that Boraró, a demiurge, multiplies game species (e.g., Tapirus terrestris and Dasyprocta aguti) in his areas. Boraró imposes rules-in-use (social institution) for the Tukano and does not allow hunting in his areas, where game can live and reproduce (Pozzobon, 2002). The Tukano respect Boraró’s will and use their information about animal behavior (local knowledge) in their management practice (management system) to avoid Boraró areas (Pozzobon, 2002). Such practice contributes to increased seed dispersal (ecological process) of trees/palms across landscapes by protected game. For example, the Inajá palm (Attalea maripa) partially depends on T. terrestris for dispersal, so the presence of T. terrestris contributes to greater abundance (ecological pattern) of Inajá in northwestern Amazonia (Fragoso et al., 2003).
In northeastern Amazonia, the Wajãpi conceive of the forest as swiddens of non-humans, which implies that when the Wajãpi open their swiddens in the forest they do so in non-human swiddens, and when they fallow their swiddens, non-humans will prepare the forest (non-human swiddens) there (Cabral de Oliveira, 2016). This aspect of Wajãpi cosmology (worldview) grounds norms and social relationships (social institutions) with these non-humans. For example, to open their swiddens, the Wajãpi cannot fell the Kapok (Ceiba pentandra), which houses Kumakajarã, its owner. The Wajãpi have a technique (management system), based on information about species characteristics (local knowledge), that consists of opening their swiddens without removing Kapok (Apina et al., 2017). This technique decreases abundances of removed species, which increases the chance of ecological drift (ecological process) in their populations (see Gilbert and Levine, 2017), so influencing random demographic modifications in tree community structure (ecological pattern). Moreover, the management practice (management system) associated with occupying/abandoning forests in space-time can increase floristic diversity (ecological pattern) by creating a regeneration mosaic across the forest (Solar et al., 2015).
In the Trombetas region, local communities known as “quilombos” are located close to stands of Brazil nut trees. Unlike many Amazonian indigenous peoples (Descola, 2005), the quilombolas (slave descendants who fled farms of the region centuries ago (Andrade, 1995)) living in these communities have a belief system (worldview) that understands nature and culture as distinct concepts. Although not exclusively, the quilombolas often use the “nature” word for differentiating non-humans from humans, who, contrary to nature, have souls (Scaramuzzi, 2016). The quilombolas say Brazil nut trees need the warmth and smell of beings with souls to produce fruits, a belief that grounds social relationships (social institution) in which the quilombolas must take care of the trees. The quilombolas use information about species characteristics (local knowledge) in their management techniques (management system) to remove vines and weeds around Brazil nut trees, which generates selection (ecological process) by enhancing fruit production (Scaramuzzi, 2016). This selection can promote opportunities for the agouti (Dasyprocta leporina, one of the main dispersers of Brazil nut (Peres and Baider, 1997)) to disperse Brazil nuts, thus increasing their sapling abundance (ecological pattern) in the region.
Amazonian river bluffs often support local communities known as “ribeirinhas”, who emerged from historical miscegenation among indigenous peoples, European colonialists, Afro-descendants and non-Amazonian immigrants (Nahum and Ferreira, 2019). In ribeirinha communities located in the Sustainable Development Reserve Piagaçu-Purus (a protected area that allows local communities to manage resources), men of the Catholic religion (worldview) have beliefs that ground norms (social institutions) for pirarucu (Arapaima gigas) management (Salgado, 2015). Unlike local Protestant men, these Catholic men cannot hunt pirarucu when they have a wound or their women are pregnant or menstruating. Catholic men thus use practices (management system) that, besides being grounded in information about pirarucu behavior (local knowledge), respect such norms and generate selection (ecological process) on pirarucu populations (i.e., oscillations in species abundance over time due to temporal changes in hunting pressure) (Salgado, 2015). Such participatory management systems have increased pirarucu abundance (ecological pattern) in riverscapes of many ribeirinha communities of central Amazonia (Viana et al., 2007; Castello et al., 2009).
These examples include sociocultural inheritance of TEK. IPLC TEK is not static ancient knowledge, but is dynamic and includes losses and innovations (Berkes et al., 2000), so that “traditional” refers to their transmission process (Carneiro da Cunha, 2009), which includes orality, practice/experimentation and observation (Levi-Strauss, 1962; Ingold, 2001). In Peruvian Amazonia, TEK transmission among the Matsigenka occurs via orality from elderly to young people, who have knowledge about plants and animals they have never seen (Shepard et al., 2001). In central Amazonia, TEK transmission about plant management among ribeirinha communities occurs mainly from parents to their children through day-to-day practices (Vásquez, 2014). In southern Amazonia, TEK transmission about fish management among quilombolas occurs through observation, so that children learn by observing local activities (Arruda et al., 2018).
The examples above show that IPLC influence landscape transformations in ways that are not normally detectable to people who neglect IPLC sociocultures. This influence occurs as worldviews ground social institutions that influence management systems that include local knowledge. Management systems, in turn, influence ecological processes that engender ecological patterns, and these influences occur from generation to generation via sociocultural inheritance.
Environmental influences on sociocultureThe examples below illustrate ecological processes and patterns influencing IPLC sociocultures that, in turn, affect environments. These feedbacks are important for continually updating TEK.
In northwestern Amazonia, precipitation, temperature and relative humidity generate abiotic selection (ecological process) on species distributions in annual cycles. Such selection influences management practices (management system) among the Tukano, who identify these cycles through observation of astronomical constellations and use them to guide their farming, hunting/gathering and fishing practices (Cochran et al., 2016). Norms influencing the execution of these practices affect game and tree abundance (ecological patterns), as shown in the previous section.
In central Amazonia, according to the Zo’é, the quatá (Ateles paniscus) want to be their partners and so they approach the Zo’é because they are attracted to the Zo’é’s aroma (Braga et al., 2020). Scientific literature about A. paniscus shows they are fearless monkeys that approach other animals to reach available resources (Youlatos, 2002), including approaches to humans (van Roosmalen, 1985). Such competitive behavior represents selection (ecological process) and has influenced Zo’é cosmology (worldview). This cosmological aspect associated with quatá approximations influences Zo’é mobility that, in turn, generates mosaics of secondary-growth forests with different floristic compositions (ecological pattern) (Franco-Moraes et al., unpublished results).
Some local knowledge differences between the Sirionó and the Ka’apor, both Tupí-Guaraní societies from Bolivian and eastern Brazilian Amazonia, respectively, emerged from the influence of different floristic patterns. The Sirionó have a smaller tree name vocabulary than the Ka’apor because forests inhabited by the Sirionó are less diverse than those inhabited by the Ka’apor. Therefore, floristic diversity (ecological pattern) influenced information about tree identification (local knowledge) in these groups and, consequently, their floristic vocabularies (Balée, 2010). In turn, Ka’apor local knowledge has influenced floristic composition (ecological pattern) (Balée and Gély, 1989).
In southern Amazonia, selection and dispersal (ecological processes) among fishes have influenced local knowledge and management. The quilombolas choose their fishing tools (management system) according to information on fish behavior (local knowledge), which includes knowledge about which fish prey on other fish/insects (selection), and which fish eat fruits and disperse their seeds (dispersal). Use of these tools permits greater efficiency in fishing, which reflects in the maintenance of fish abundances (ecological pattern) (Arruda et al., 2018).
In northeastern Amazonia, phenology (ecological pattern) influences management among the quilombolas of the Ipaú-Anilzinho Extractive Reserve (a protected area that allows local communities to manage resources). Hunting and fishing practices (management system) occur during the fruiting and flowering period of certain trees, respectively, according to game and fish abundances. This hunting/fishing rotation is important for the maintenance of game and fish diversity (ecological pattern) through time (Figueiredo and Barros, 2016).
The examples above show how abiotic conditions and biotic interactions (ecological processes), as well as floristic diversity and phenology (ecological patterns bequeathed by previous generations, i.e., ecological inheritances) have influenced Amazonian IPLC worldviews, management systems and local knowledge. Management systems, in turn, have modified ecological processes and patterns, thus generating sociocultural–environmental feedbacks.
The historical backgroundSince landscape transformations are influenced by historical events (Balée, 2009; Crumley, 2017; Walters et al., 2019), cultural niche construction is better understood with the role of history in mind. The examples below show how Amazonian sociocultural–environmental feedbacks have been contingent upon history.
In eastern Amazonia, an ancient local war led the Parakanã to split into two groups. Thereafter, a sequence of events modified the socioculture of both Parakanã groups. While the western group intensified conflicts, expanded its action area and adopted a hunter-gathering lifestyle, the eastern group adopted political centralization, isolated itself and adopted a sedentary-horticultural lifestyle (Fausto, 2001). Therefore, a war triggered changes in social relationships (social institution) and management practices (management system) among the Parakanã (Fausto, 2001).
Colonization of Amazonia during the 17th–19th centuries affected all indigenous societies. For example, because Jesuit missions used Ka’apor labor to collect cacao (Theobroma cacao), the Ka’apor stopped managing Theobroma subincanum (a pre-Colombian practice) and replaced it by cacao in their landscapes. Therefore, a colonization event introduced changes in information about species characteristics (local knowledge) and management practices (management system) among the Ka’apor, which modified local floristic composition (ecological pattern).
Colonization also influenced the emergence of Amazonian local communities. In the 18th century, in order to develop Amazonia and create a local identity, the Portuguese court ordered the miscegenation between colonists and indigenous peoples, and their descendants were enslaved to work in the cultivation of cacao, coffee (Coffea sp.), and other crops (Nahum and Ferreira, 2019). Years later, African slaves were introduced to work in sugar cane (Saccharum officinarum) plantations. During that period, both local and African descendants fled to riverbanks and established local communities today known as ribeirinhas and quilombolas, respectively (Nahum and Ferreira, 2019). Landscape transformations among these local communities can best be understood by considering local colonial history. For example, colonial and indigenous cultures influenced ribeirinhos and quilombolas in their hunting norms (social institutions), swidden-fallow cultivation practices (management system) and information about animal behaviors (local knowledge) (Adams et al., 2008).
Migration has influenced Amazonian IPLC societies in many contexts. During their migration to Bolivian Amazonia, the Sirionó abandoned their farming practices due to local conflicts, which changed their lifestyle from a sedentary/horticultural to a hunter-gatherer pattern (Balée, 2013). Therefore, a migration event influenced changes in Sirionó management practices (management system), which influenced the way the Sirionó interact with ecological processes and patterns (Balée, 2013).
During 1877–1912, with the growing demand for rubber in the world market, thousands of people from northeastern Brazil (known as “nordestinos”) migrated to work in latex extraction from the rubber tree (Hevea brasiliensis) in Brazilian Amazonia (Nahum and Ferreira, 2019). After the rubber boom collapsed, the nordestinos remained in Amazonia and learned IPLC sociocultures, including their swidden-fallow cultivation practices (management system) and information about species distribution (local knowledge) (Nahum and Ferreira, 2019). Therefore, a migration event influenced sociocultural changes in northeastern Brazil migrants by introducing new sociocultural–environmental interactions for these people, which influenced rubber abundance (ecological pattern).
The examples show that wars, colonization and migrations have influenced changes in Amazonian IPLC social institutions, management practices and local knowledge with consequent changes in local ecological processes and patterns. Moreover, the examples show that historical events were responsible for creating Amazonian local communities, which were influenced by colonists’ and indigenous peoples’ sociocultures.
Application of the frameworkIn our examples, we have shown that cosmologies, religions and belief systems (worldviews) ground hunting norms, social relationships and territorial rules (social institutions). Social institutions, in turn, guide fish management, swidden-fallow cultivation and the choice of trees to be cut (management systems). Management systems include information about animal behavior and species identification, distribution and diversity (local knowledge), and are responsible for selecting game and trees, and dispersing seeds (ecological processes). These processes, in turn, modify species composition, abundance and diversity (ecological patterns). Our examples also show that abiotic conditions and animal behaviors (ecological processes) influence cosmologies (worldview) and management practices (management systems). Moreover, floristic diversity and temporal patterns of fruiting/flowering (ecological patterns) influence species identification (local knowledge) and hunting/fishing practices (management systems). We urge scientists, conservation practitioners and policymakers to consider these sociocultural–environmental feedbacks in their research, conservation strategies and public policy planning involving IPLC. Below, we highlight how our framework can be useful in these cases (Appendix A lists guiding questions that can be asked when using our framework).
First, our framework can be useful for scientists. Scientists without training in anthropology often neglect IPLC sociocultures in environmental assessments because they consider that IPLC TEK is spiritual knowledge (Albuquerque et al., 2020). We highlight the importance of considering IPLC sociocultural elements, such as worldviews and social institutions, in environmental assessments, and our framework can help scientists to identify these elements and understand how they influence management systems (see questions 1–8 in Appendix A). The framework shows that management systems, in turn, modify ecological patterns through four groups of ecological processes, so that efforts can be made to identify such processes and to investigate how they influence patterns (see questions 9–12 in Appendix A). The framework also can be useful to investigate how ecological processes and patterns have influenced IPLC sociocultures, as well as which sociocultural elements have been influenced by historical events (see questions 13–16 in Appendix A). In this sense, when using our framework, collaborations with IPLC, as well as between social and natural scientists, can identify information that is not normally detectable to people who neglect IPLC perspectives. In Australia (Austin et al., 2019) and Brazil (Pimenta et al., 2018), such collaborations have identified sociocultural–environmental feedbacks through partnerships that involve mutually beneficial goals, methods and interpretation of results. Finally, our framework highlights the importance of scientists accounting for the historical events responsible for modifying interactions between IPLC sociocultures and their environments. Such accounting can increase our understanding about landscape environmental changes, as well as offering a historical reference to assess modern ecological processes and patterns (Balée, 2006; Swetnam et al., 1999).
Second, our framework can be useful for conservation practitioners. Some conservation practitioners have suggested that nature should be as isolated as possible from humans for better conservation (see Cafaro et al., 2017; Kopnina et al., 2018; Pimm et al., 2018). They disregard the fact that most landscapes targeted by conservation efforts are inhabited by IPLC (Maffi and Woodley, 2012; Pearce, 2016; Clement et al., 2020), and that more effective and ethical outcomes can be achieved through biocultural conservation, which is conservation of biodiversity and sociocultural diversity (Maffi and Woodley, 2012; Gavin et al., 2015). Our framework can help to identify sociocultural–environmental interactions that can contribute to biocultural conservation (see questions 17–20 in Appendix A). For this, it is essential that conservation strategies consider the sociocultural and environmental inheritances associated with these interactions, focusing not only on ecological processes and patterns, but also on how local worldviews, social institutions, management systems and local knowledge are associated with such processes and patterns. This requires the participation of IPLC in conservation strategy planning, so that species, communities and landscape conservation occur in accordance with local socioculture demands (Kohler and Brondizio, 2017). In Ghana, for example, conservationists have suggested that the Kumawu cosmology can be synergized with scientific conservation practices to protect landscapes of the Bomfobiri Protected Area (Adom, 2018). When IPLC and conservation practitioners disagree about conservation strategies (see, for example, Kopnina, 2020; but also see Suter, 2020), understanding how local sociocultural–environmental interactions occur can help in planning strategies with mutual agreement.
Third, our framework can be useful for policymakers. Policymakers often formulate public policies that disregard the ways IPLC interact with their environments. For example, in Borneo, policymakers forced the Dayak to live in a narrow part of their original territory, which led to land use intensification that compromised the resilience of Dayak swidden-fallow systems (Setyawan, 2010). To avoid sociocultural injustice and environmental degradation, the Intergovernmental Platform on Biodiversity and Ecosystem Services (IPBES) has supported the inclusion of IPLC in public policy planning about biodiversity conservation (Díaz et al., 2018). In this context, our framework can be useful for policymakers that aim to elaborate biocultural conservation policies including IPLC stewardship and governance. For this, public policies (e.g., territorial demarcation, health care, school education and territorial/resource inspection) need to be formulated considering local worldviews and social institutions (Fernández-Llamazares and Cabeza, 2017; Albuquerque et al., 2020), as well as local landscape transformation dynamics (Armstrong and Veteto, 2015; Swetnam et al., 1999). Our framework can help to identify sociocultural–environmental interactions involved in such dynamics (see questions 21–25 in Appendix A). Our framework also highlights the importance of considering local historical events when identifying these interactions. Understanding the historical background of IPLC’ landscape transformations is crucial to comprehend current sociocultural–environmental interactions that favor biocultural conservation.
Final remarksWe argue that understanding IPLC’ landscape transformations can help scientists, conservation practitioners and policymakers to generate scientific knowledge, biodiversity conservation and IPLC’ well-being. For this, the first step is to ensure IPLC’ rights, thus generating biocultural conservation through IPLC stewardship and governance. In Brazilian Amazonia, for example, indigenous peoples (Article 231 of the 1988 Constitution) and local communities (Decree 6.040 of 2007) have rights to live in their traditional territories and practice their livelihoods (but see Fraser, 2018). Ensuring these rights requires respecting local sociocultural–environmental interactions, as well as the historical dynamics of landscape transformations in the region. In fact, ensuring IPLC’ rights protect not only IPLC but also biodiversity. For example, biodiversity on indigenous lands equals that in protected areas (Schuster et al., 2019), and indigenous lands present at least equivalent conservation results with a fraction of the budget of protected areas (Tauli-Corpuz et al., 2018). Finally, we must remember that IPLC’ land rights must be guaranteed not only because IPLC are useful to global society, but also because they, as well as all non-human beings ecologically interconnected with their sociocultures, have the right to live there.
Declaration of interestsThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
JF-M thanks CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior do Brasil) for a doctoral scholarship; AAO and CRC thank CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil) for research fellowships (process numbers 302128/2018-2 and 303477/2018-0, respectively). We thank Patrícia Torres (USP), Gilton Mendes (UFAM) and Rui Murrieta (USP) for suggestions and criticisms on the first draft of this manuscript.
| To be asked by scientists in their research: |
| 1 – What are the local cosmologies, belief systems and ethics (worldviews) that can be associated with the scientific inquiry? |
| 2 – How do worldviews influence local social institutions? |
| 3 – How do these social institutions relate to the use of local resources and environmental conditions? |
| 4 – How is the use and management of local resources done? |
| 5 – How do social institutions provide the base for local management systems? |
| 6 – What are the types of environmental manipulative behaviors of the management system associated with the scientific inquiry? |
| 7 – What local knowledge is the basis for these manipulative behaviors? |
| 8 – How are sociocultural inheritances transmitted from generation to generation? |
| 9 – Which ecological processes are influenced by the management system? |
| 10 – Which ecological patterns are influenced by such processes? |
| 11 – How do such processes influence ecological patterns? |
| 12 – What are the ecological inheritances bequeathed by previous generations in the current ecological patterns? |
| 13 – How have abiotic (e.g., environmental conditions) and biotic (e.g., animal behaviors) ecological processes influenced the four levels of socioculture? |
| 14 – How have ecological patterns (e.g., floristic and animal diversity) influenced the four levels of socioculture? |
| 15 – What kinds of historical events may have influenced local socioculture? |
| 16 – How do these historical events modify local relations among sociocultural and environmental elements? |
| To be asked by conservation practitioners in the formulation of their conservation strategies: |
| 17 – How do local worldviews and social institutions (norms, rules etc.) influence local biodiversity conservation? |
| 18 – How do management systems contribute to the maintenance of ecological patterns, such as the abundance of endangered species and diversity of communities? |
| 19 – How can local knowledge contribute to scientific conservation practices and vice-versa? |
| 20 – From the answers to questions 15 and 16: how do these changes influenced local biodiversity conservation? |
| To be asked by policymakers in their public policy planning: |
| 21 – How can local worldviews, associated with, for example, territorial mobility, contribute to the management and inspection of local territory? |
| 22 – How can social institutions contribute to local governance? |
| 23 – How can management systems contribute to landscape stewardship? |
| 24 – How do ecological inheritances contribute to international governmental agreements on biodiversity conservation (e.g., changes in forest structure or abundance of endangered species)? |
| 25 – From the answers to questions 15 and 16: how can public policies be reformulated to meet demands associated with new forms of local interactions (e.g., interactions among social institutions and ecological processes modified by historical events)? |
According to Convention 169 of the International Labour Organization, indigenous peoples are those who identify themselves as indigenous and “descent from the populations which inhabited the country, or a geographical region to which the country belongs, at the time of conquest or colonization or the establishment of present state boundaries and who, irrespective of their legal status, retain some or all of their own social, economic, cultural and political institutions”; local communities (or tribal communities) are those who identify themselves as tribal and are peoples “whose social, cultural and economic conditions distinguish them from other sections of the national community, and whose status is regulated wholly or partially by their own customs or traditions or by special laws or regulations”.
TEK is “a cumulative body of knowledge, practice, and belief, evolving by adaptive processes and handed down through generations by cultural transmission, about the relationship of living beings (including humans) with one another and with their environment” (Berkes et al., 2000:1252).