In riparian forests, width contributes most importantly to maximizing diversity. Therefore, corridors with different widths should differ in richness, abundance, and composition. We tested this hypothesis for the bird communities of two forests on the Upper Paraná River floodplain, Paraná, Brazil. Richness and abundance were higher in riparian forest with mean width of 50m in each margin and lower anthropogenic disturbance. Species diversity increased 30%, with increase in total width from 40m to 100m on average. Bird species composition also differed, and groups with the strictest ecological requirements were better represented in the wider, better-preserved forest. This indicates that conservation of riparian forests has a positive effect on their bird communities. We suggest that these environments are prioritized for recuperation, and that a 50m width on each side of a stream is necessary for riparian forests to effectively fulfill their function in the landscape. We also note that the recently discussed Brazilian Forest Code does not conform to this requirement.
© 2014 Associação Brasileira de Ciência Ecológica e Conservação. Published by Elsevier Editora Ltda.
In fragmented landscapes, the survival of species depends on their ability to persist in fragments and/or move across the landscape (Lees & Peres 2008). Riparian forests are important corridors for many biological groups in fragmented landscapes, because they promote increased connectivity and hence species richness and flow of individuals (Lees & Peres 2008). These corridors increase genetic variability (Vieira & Carvalho 2008) and local biodiversity (Anjos et al. 2007), reduce climatic variations and their consequences (Marini et al. 2009), and allow forest organisms of adjacent biomes to disperse (Silva 1996). Riparian corridors are essential to maintain the diversity of plant and animal communities in many biomes, particularly in the Atlantic Forest (e.g., Metzger et al. 1997; Anjos et al. 2007).
The available forest area along bodies of water is an important factor affecting the richness and species composition of a wide variety of organisms (Vieira & Carvalho 2008; Tubelis et al. 2004). The width of riparian corridors is the most important factor benefiting biodiversity, and maximizing this width improves habitat quality by reducing edge effects (Metzger 2010). Other factors including the length, continuity, and degree of conservation of corridors (Lees & Peres 2008); and the surrounding matrix type, topography, and extent of the areas of riparian influence (Metzger et al. 1997) also influence the quality of riparian corridors.
The protection of these corridors, although present in the former Brazilian Forest Code (Federal Law No. 4.771 of September 15, 1965), was not effective. It is common to observe properties that contravene it, where the permanent preservation areas (PPA) along streams are fully or partially occupied (Sparovek et al. 2011). According to this law, riparian forests on the margins of streams up to ten meters wide should be 30m in width on each side, but the new Forest Code provides for regularization of deforestation in areas of consolidated use, with reconstitution of the PPA, according to farm size. This reconstitution would be negligible, ranging from five meters on each side to a maximum of 15m, and still allowing the use of these areas for activities of agroforestry, ecotourism, and rural tourism. Thus, the recovery of the functionality of this environment is compromised, since there will be no more incentive to restore them and their use and exploration will still be allowed (Sparovek et al. 2011).
In this context, we evaluated two riparian forests of different widths, with respect to the richness, composition, and abundance of their bird species. We tested the following hypotheses: the wider riparian forest (with less anthropogenic disturbance) will support greater species richness and abundance of individuals than the narrower forest (with greater anthropogenic disturbance); and species composition will differ between the forests, despite their spatial proximity (6km apart). If these assumptions are correct, narrow riparian forests have limited importance for biodiversity conservation of forest birds in areas where loss of forest habitat is extreme and most remnants are restricted to the margins of water bodies.
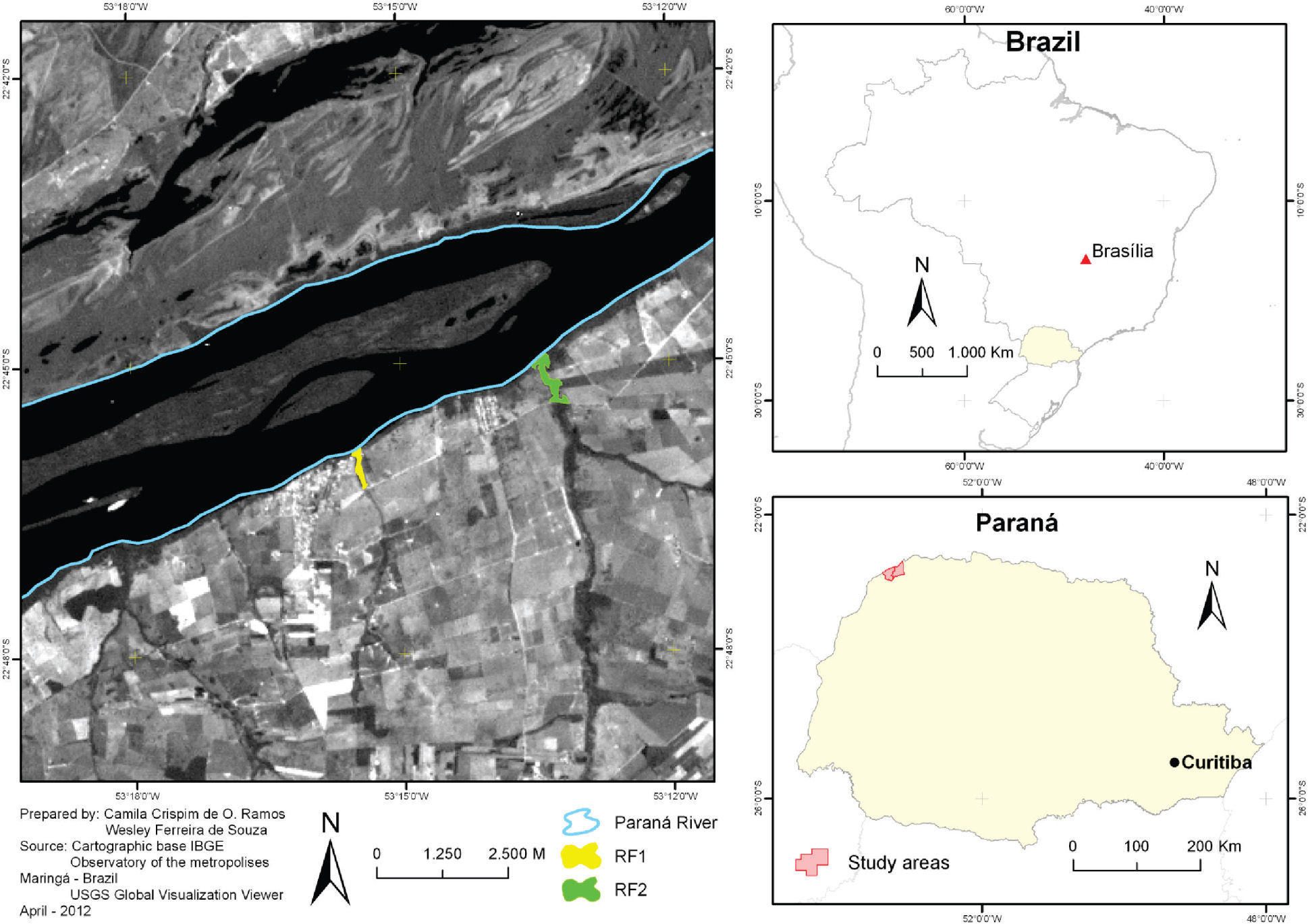
MethodsStudy areasThe study was conducted on the Upper Paraná River floodplain (UPR) in northwestern Paraná state, Brazil. This area is a transitional zone of the Atlantic Forest with the Cerrado (Mendonça et al. 2009). The riparian forests studied are west of the Paraná River, at an altitude of approximately 260m, bordering the Caracu stream (22°45’55” S and 53°15’30” W, 4.5ha), and the São Pedro River (22°44’58” S and 53°13’24” W, 11ha) (Fig. 1). Both forests were intensively exploited and degraded by farming and urbanization, but in the last decade were fenced and allowed to regenerate naturally. The regional climate, according to the Köppen-Geiger system, is Cfa (humid subtropical mesothermal) with an annual mean temperature of 22°C (summer mean 26°C and winter 19°C) and mean annual rainfall of 1,500mm. However, in some years, the climate may be Cwa (high-altitude tropical) with a tropical rainfall pattern and dry winters (Maack 2002). The vegetation is semideciduous seasonal forest (western boundary of the Atlantic Forest), which now covers only 1% of its original extent and occurs in a few small fragments and riparian remnants along the Paraná River and its tributaries (Campos & Souza 1997).
The first riparian forest (RF1) averages 40m wide (20m on each side of the stream), but in some places narrows to 15m in total. RF1 extends along the Caracu stream in the municipality of Porto Rico, between urban and rural areas. It is a discontinuous secondary forest, with a relatively open understory and some areas occupied by vines and lianas. The second riparian forest (RF2) averages 100m wide (50m on each side) in its narrower stretches, but the total width exceeds 100m in several parts. This forest extends along the São Pedro River in the municipality of São Pedro do Paraná, in a rural area. It is a continuous secondary forest, with an open understory along one bank, and denser growth with tangles of vines and lianas on the opposite bank. RF2 has a better conservation level and lower anthropogenic disturbance.
Field methodWe obtained the field data along a transect 850m in length through the two riparian forests, starting at the point where each stream enters the Paraná River. Data were gathered from September to November 2008, using the point count method (Blondel et al. 1981) as adapted by Anjos (2007) for studies of forest fragments, which was sufficient to detect 75% of forest species diversity in these sites. Samples were taken monthly on four consecutive days, two in each forest. We allocated four points along predetermined trails in each forest, 200m distant from each other and 100m from the beginning of the transect. We began sampling at dawn and ended after four hours. Each point was sampled twice in the morning, in the sequence 1, 2, 3, 4, and then 4, 3, 2, 1. The next morning, the order was reversed. We remained for 15min at each point, performing observations, with a 15-min interval between points. We included visual and auditory records of the species present within a radius limit, taking care not to record species outside the forest; each couple or group (for social species) was considered a contact. We recorded unrecognized vocalizations with a portable recorder and unidirectional microphone for later identification at the Laboratory of Ornithology and Bioacoustics, Universidade Estadual de Londrina. From the quantitative survey data, the point abundance (IPA) for each species was calculated by dividing the number of contacts by the total number of points sampled in each area (Blondel et al. 1981).
Procedures for analysisWe included only species that are most dependent on forest habitats, according to Parker III et al. (1996); birds that live in the habitat matrix and are occasionally recorded in the forest edge were not included, e.g., Crotophaga ani, Mimus saturninus, and Furnarius rufus. We also excluded nocturnally active birds (families Strigidae, Caprimulgidae, and Nyctibiidae), and the families Accipitridae, Falconidae, and Throchilidae because of their high mobility and/or different habits.
To evaluate differences in species composition between the forests, the species were classified in different groups based on data for habitat use, food habits, endemism, and distance of the UPR from the geographic distributional limit of the species. Regarding habitat use, we included strictly forest species, those that only occur in forest formations as defined by Parker III et al. (1996). Regarding food habits, we classified the species as frugivores, insectivores, or omnivores according to Anjos & Schuchmann (1997), using the frugivore classification of Mendonça et al. (2009). We considered endemic species to be those restricted to the Atlantic Forest or to central South America, according to Parker III et al. (1996).
Statistical proceduresWe evaluated whether the sampling effort was sufficient to obtain a representative number of bird species in each forest through accumulation curves and estimates of species richness. The abundance-based coverage estimator (ACE) was used, because it considers abundant species, i.e. those represented by more than ten individuals, besides considering singletons and doubletons (Lee & Chao 1994). Rarefaction curves of species for each area were constructed to evaluate the difference in richness between the areas. For both analyses, we used the software EstimateS 7.5.2 and Statistica 7.0. We used the G test with a correction factor to test for differences between the number of contacts (corrected for sampling effort, i.e., IPA x 100) of each species in both forests. When the expected number of contacts was less than five, we calculated the exact probabilities for the binomial test; the value of α was set at 0.01 for both tests.
We ordered the sampling points in RF1 and RF2 based on species composition and abundance. We used a correspondence analysis (CA) by assigning less weight to rare species, so they would not affect the ordination. As a criterion for retention and interpretation of the CA axes, we used only axes with eigenvalues greater than 0.20, as recommended in the literature (Manly 2008), and used PC-ORD 3.5 to perform this analysis. The scores from this analysis were submitted to a univariate analysis of variance (ANOVA) to investigate possible differences in these community structures. The community structure based on each particular group (habitat use, food habits, endemism, and distance from the distributional limit), was evaluated by the scores of species in parametric ANOVA, and by a nonparametric test (Kruskal-Wallis) when the conditions for the ANOVA were not satisfied; both tests were performed with Statistica 7.0. The full summary of the research papers and software packages used in statistical methodology are provided in the supplementary material online.
ResultsIn the two riparian forests, we recorded a total of 74 species of forest birds: 49 in RF1 and 70 in RF2. The accumulation curve and estimation of species richness indicated that data from RF1 were closer to the asymptote, while in RF2, richness still tended to increase with sampling effort (Fig. S1A, supplementary material online). Rarefaction curves showed that richness (independently of abundance) in RF2 was higher than in RF1 (Fig. S1B, supplementary material online). Four species were unique to RF1 and 25 were unique to RF2. Among the 45 species common to both areas, four were more abundant in RF1, 14 more abundant in RF2, and 28 showed similar abundances (Table S1, supplementary material online).
In the correspondence analysis, the riparian forests formed two groups, separating the RF1 and RF2 sampling points (Fig. 2A). Only the first CA axis was retained for interpretation (Table S2, supplementary material online). The sampling areas differed significantly, as confirmed by the analysis of variance from the scores generated for axis 1 (F11, 84=16.47, p<0.001, Fig. 2B).
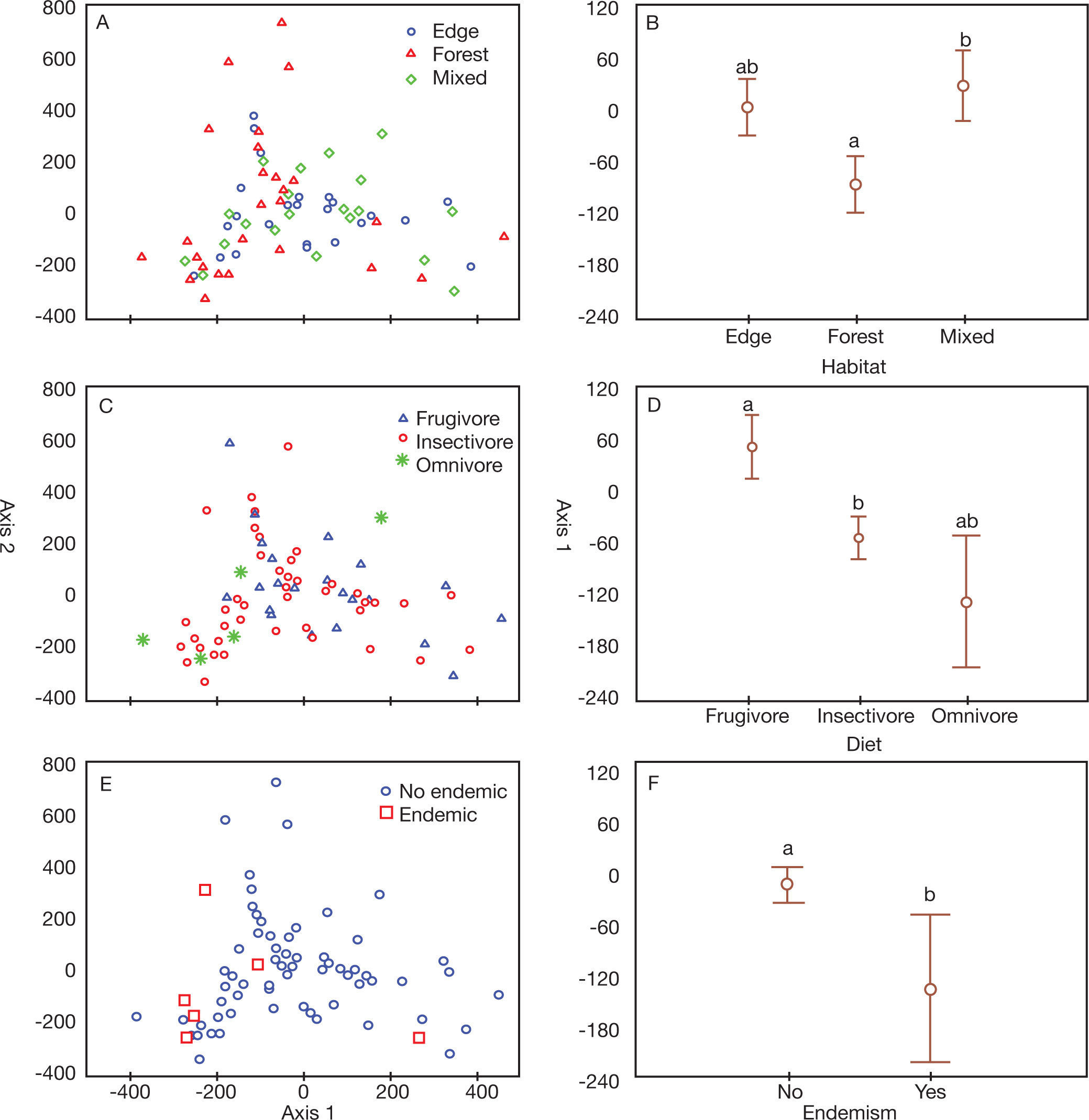
To evaluate how these communities were structured according to the species characteristics, we used the scores generated for the species, relative to axis 1, to construct the graphs. These graphs show how each group of species are organized in the space in a direct relationship with the ordination of the sample points. Regarding the use of habitat (Fig. 3A), edge species predominated in RF1, and forest species in RF2 (KW-H2, 74=7.26, p<0.05, Fig. 3B).
Graphical representation of scores generated for species grouped by preferential habitat (A), through the ranking of point samples from RF1 and RF2 and graphs of the variance analysis for the axis retained (Axis 1), with (B) scores of species grouped by habitat preference; graphical representation of scores generated for species grouped by feeding habit (C), through the ranking of point samples from RF1 and RF2 and graphs of the variance analysis for the axis retained (Axis 1), with (D) scores of species grouped by feeding habit; graphical representation of scores generated for species grouped by the presence of endemism (E), through the ranking of point samples from RF1 and RF2 and graphs of the variance analysis for the axis retained (Axis 1), with (F) scores of species grouped by the presence of endemism.
Regarding feeding habits (Fig. 3C), insectivorous species predominated in RF2, and frugivores in RF1 (KW-H2, 74=7.76; p<0.05, Fig. 3D).
Finally, endemic species (Fig. 3E) predominated in RF2 (KWH1, 74=4.20, p<0.05, Fig. 3F).
DiscussionThe data from this study demonstrated that the difference in forest width, combined with different degrees of conservation of the riparian corridors, altered the richness, abundance, and species composition of forest birds. The ability of these riparian forests to support groups of species with stricter ecological requirements increased when both aspects of these particular forest formations (width and anthropogenic disturbance) were optimized.
Forest bird species diversity increased 30%, with increase in total width from 40m to 100m on average. Studies of arboreal vegetation in the Atlantic Forest showed that corridors less than 100m wide have limited effectiveness in maintaining diversity (Metzger et al. 1997). For birds in the Cerrado, Tubelis et al. (2004) postulated that the minimum width of native vegetation should be 120m. According to the authors, this range allows the conservation both of birds associated with riparian forests, and those that are dependent on adjacent savannas.
The community composition found in RF1, where edge species predominated, mainly reflects the influence of the forest width on the community structure, although other factors, such as the intense human presence from the adjacent urban landscape and the resulting lower biotic integrity, likely also had an important role in the results obtained. The width of riparian forest affects habitat quality and regulates the area impacted by edge effects, i.e., the microclimatic changes and the increase in disturbances at the edges of these habitats (Metzger 2010). According to Metzger, these effects can vary in extent depending on the species and processes in question, and also according to the physical characteristics of the site, in particular the solar direction, latitude, and adjacent matrix type, which influence the amount of incident solar radiation. In general, stronger effects occur in the first 100m (Laurance et al. 2002), implying that corridors less than 200m wide are essentially highly disturbed edge environments. Strictlyforest species would need corridors at least 200m wide (Lees & Peres 2008).
In this study, examination of three parameters indicated that the better-preserved riparian forest harbors a set of species with stricter ecological requirements. The first parameter, which takes into account habitat use, indicated that the presence of a higher proportion of strictly-forest species in RF2 indicates that it is a higher-quality habitat. Greater biotic integrity and a less-disturbed surrounding landscape allow the occurrence of more-specialized groups such as Dendrocolaptidae, which have stricter ecological requirements, according to Poletto et al. (2004).
The second parameter analyzed was the preferred feeding habit of the species, and we found that insectivores were more concentrated in RF2. Insectivores, especially those that forage in lower strata, are considered to be the most vulnerable species by many investigators (Ribon et al. 2003). Considering that this area also harbored more strictly-forest species and that many groups of insectivores have relatively limited mobility through the landscape (e.g., Lees & Peres 2009), these characteristics probably act to concentrate most species that are sensitive to fragmentation in this area. According to Uezu & Metzger (2011), to understand the sensitivity of species to habitat fragmentation, it is necessary to consider the multiple dimensions of features of the species.
We found a higher prevalence of endemic species - the third parameter analyzed - in RF2, which provides evidence that for the maintenance of species dynamics in a particular region, the quality of the corridors is a key aspect.
This study demonstrated that maintaining riparian forests intact can have a positive effect on bird conservation. The data presented here suggest that in the case of streams (where the requirement, according to the Forest Code, Law 4.771/65, is 30m on each side), the PPAs should be expanded to a minimum of 50m on each side of a stream, to aid in conserving species with stricter ecological requirements.
AcknowledgmentsWe thank Dilermando Pereira de Lima Júnior and Nadson Ressié Simões for revision of this manuscript. We are also grateful for the cooperation of the staff of Laboratório de Ornitologia e Bioacútica - UEL and Núpélia-UEM. Financial support was provided by CNPq (process no. 130294/2008-0), PELD - Site 6 and PEA/UEM.
Supplementary materialSupplementary material associated with this article can be found, in the online version, at www.naturezaeconservacao.com.br.