Reintroduction can be enhanced by data from long-term post-release monitoring, which allows for modeling opportunities such as population viability analysis (PVA). PVA-relevant data were gathered via long-term monitoring of reintroduced red-billed curassows at the Guapiaçu Ecological Reserve (REGUA), located in Rio de Janeiro, Brazil, over 25 months. In the present article, we (1) assess the robustness of the reintroduction plan, (2) evaluate the viability of the current reintroduced population, and (3) examine mitigation options to increase the viability of this population. VORTEX indicates that the initial plan, fully implemented, was likely to establish a viable population at REGUA. The current population is unviable; the best mitigation strategies are to eliminate hunting altogether, or at least reduce it by half, and to supplement ten immature pairs in 2015. A positive long-term outcome at REGUA is still possible; we encourage the Brazilian government and private stakeholders to consider population supplementation, both to achieve success at REGUA and to improve the evidence base for future reintroductions.
The main goal of any species reintroduction program for conservation purposes should be to establish a self-sustaining wild population, defined as one with high probability of persistence and positive stochastic growth rate (Schaub et al. 2004). Evaluating the success of reintroduction programs requires good data from long-term post-release monitoring (Scott & Carpenter 1987), as these allow for modeling opportunities such as population viability analysis (PVA; Beissinger & Westphal 1998).
Fewer than ten natural populations of the red-billed curassow Crax blumenbachii, a cracid species (IUCN status ‘Endangered’, BirdLife International 2012) endemic to the Brazilian Atlantic rainforest, persist in the wild, in the states of Bahia and Espírito Santo (IBAMA 2004). Between 2006 and 2008, 46 radio-tagged birds, supplied by the CRAX Brasil breeding center in Belo Horizonte, were released into the Guapiaçu Ecological Reserve (REGUA), in the state of Rio de Janeiro. Systematic long-term monitoring for 25 months enabled the collection of PVA-relevant data on survival, home range size, social interaction, and habitat selection (Bernardo et al. 2011a; 2011b).
The project at REGUA was the first to include post-release monitoring for this species. Three other reintroductions, in different sites in the state of Minas Gerais during the 1990s, also involved birds from CRAX Brasil (Azeredo & Simpson 2004); fourth-generation breeding of wild-born birds is reported to have occurred at one site (Fazenda Macedônia; R. Azeredo, pers. comm.). The initial plan for REGUA was the release of 100 birds, in groups of 20 individuals per year, over a period of five years (2006–2010). These figures were based on evidence that the chances of establishing a self-sustaining free-ranging population and improving reproduction and survival rates increase with the initial founder population size (Fischer & Lindenmeyer 2000; Armstrong & Seddon 2007). However, in early 2009, when fewer than half the projected number of birds had been released, unforeseen circumstances curtailed the supply of birds. Despite a relatively high survival probability compared to other reintroduced galliforms (75%; Bernardo et al. 2011b), the initial population (n=46, with a sex ratio of 2:3 males to females), was possibly too small for a viable population in the long term. In the present article, we (1) assessed the robustness of the initial plan, which was the release of 100 individuals over five years, (2) evaluated the viability of the current population at REGUA, and (3) examined which mitigation option might increase the viability of the surviving reintroduced population.
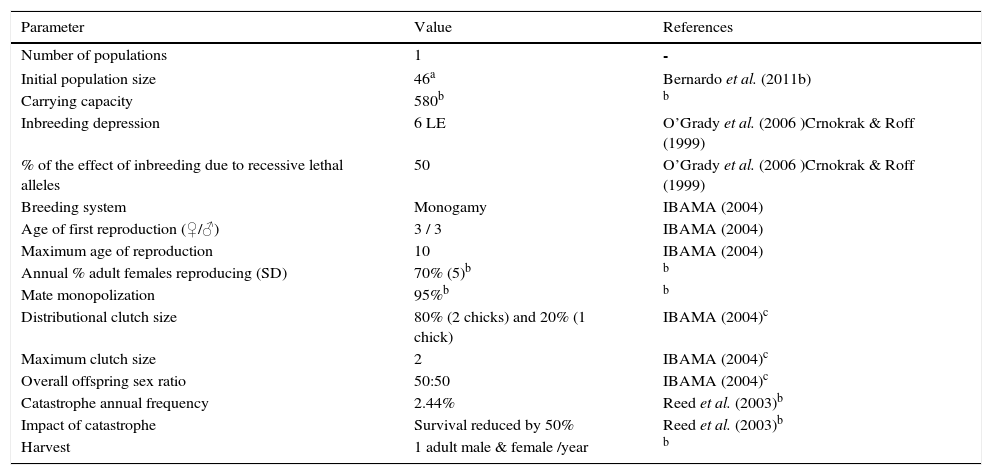
Materials and methodsPopulation viability analysisFor the PVA, we used the software VORTEX version 9.9b (Miller & Lacy 2005); earlier versions of this software have been widely used to model wildlife populations and, when tested against long-term field study datasets, produced accurate predictions (Brook et al. 2000). Population attributes (e.g. breeding success, clutch size, sex ratio at birth, initial population size) were determined mostly based on IBAMA (2004), Azeredo & Simpson (2004), and Bernardo et al. (2011a) (Table 1). We considered a population viable if its probability of extinction in 100 years was<40%. We created three scenarios: (1) “initial plan”: the situation that should have resulted had the project not been modified; (2) “current population”: the situation that developed in 2006–2008; and (3) “strategic mitigations”,: the options for guaranteeing long-term persistence of the current population.
Gender, age, marital status and ethnicity of Bauru’s donors registered in REDOME (n=3542).
| Parameter | Value | References |
|---|---|---|
| Number of populations | 1 | - |
| Initial population size | 46a | Bernardo et al. (2011b) |
| Carrying capacity | 580b | b |
| Inbreeding depression | 6 LE | O’Grady et al. (2006 )Crnokrak & Roff (1999) |
| % of the effect of inbreeding due to recessive lethal alleles | 50 | O’Grady et al. (2006 )Crnokrak & Roff (1999) |
| Breeding system | Monogamy | IBAMA (2004) |
| Age of first reproduction (♀/♂) | 3 / 3 | IBAMA (2004) |
| Maximum age of reproduction | 10 | IBAMA (2004) |
| Annual % adult females reproducing (SD) | 70% (5)b | b |
| Mate monopolization | 95%b | b |
| Distributional clutch size | 80% (2 chicks) and 20% (1 chick) | IBAMA (2004)c |
| Maximum clutch size | 2 | IBAMA (2004)c |
| Overall offspring sex ratio | 50:50 | IBAMA (2004)c |
| Catastrophe annual frequency | 2.44% | Reed et al. (2003)b |
| Impact of catastrophe | Survival reduced by 50% | Reed et al. (2003)b |
| Harvest | 1 adult male & female /year | b |
Data on key natural history parameters (Azeredo & Simpson 2004; IBAMA 2004; Lima et al. 2008; Bernardo et al. 2011a; 2011b) were sufficient for constructing the models. However, future research should focus on chick mortality and female breeding rates in order to enhance model accuracy. Data deficiencies need not affect results when the goal of PVA is comparative (Akçakaya & Sjögren-Gulve 2000). We ran 10,000 iterations for each scenario.
The size of released populationsFor the “initial plan” scenario, we considered an initial population size of 20 immature (2–3 years) individuals (ten males, ten females) and a supplementation of ten immature pairs every year over five years. For the “current population” scenario, we considered an initial population size of 46 individuals released in 2006–2008 (26 females, 20 males) (Bernardo et al. 2011b). Since they were released in different years, in 2009 they had different ages (Table 1). For the “strategic mitigation” scenario, we considered the values adopted for the “current population” scenario, but with further supplementations of ten immature pairs at six and 12 years after cessation of releases (i.e., in 2015 and 2021), or one immature pair/year over the next ten years.
Labels and state variablesWe created an individual state variable (IS1) in order to differentiate the mortality rates of reintroduced birds from those of supplemented birds every year a supplementation occurs. We entered an initialization function that defined the starting value of IS1 at the beginning of the simulation as (V>(2x))*(Z>(2x)), where x=initial population size, V=paternal allele and Z=maternal allele. This means there will be two alleles for each bird (one paternal and one maternal) in the initial population, and that all offspring, before any supplements are added, will have alleles with totals 2x or smaller. Supplemented birds will have new alleles with totals higher than 2x. The initialization function is only applied to the initial animals in the population, and will be set to 0 for the initial animals (0*0) and to 1 for any supplemented animals (1*1). IS1 will remain 0 for all non-supplements (0+1)*0, and will continue to increase for all supplements.
We also entered a function that defined IS1 at birth (birth function=0), as well as a function that defined IS1 each year (transition function: = (IS1+1)*IS1). This means that immediately after supplementation, IS1 will change from 1 to 2 ((1+1)*1). Thus, in the year following supplementation, IS1=2 (when supplemented birds undergo differential mortality). This value is then used to identify the first year that the population will be supplemented.
Carrying capacity (K)Since paired adult curassows occupy a mean home range of 250ha (Bernardo et al. 2011a), we assumed a family unit (adult pair with one to two young) would occupy the same area. Based on 48,270ha of suitable habitat at REGUA (CSSB unpubl. data), we assumed a minimum carrying capacity of ~580 individuals, i.e., (48,270/250)×3. Home ranges are not necessarily defended as territories; therefore, overlaps would allow for higher overall numbers. To check whether carrying capacity influences population viability results (whether K is a sensitive parameter), we also modeled the “initial plan” and “current population” scenarios by considering K=772 individuals, i.e., (48,270/250)×4. For the “strategic mitigation” scenario, we considered K=580 individuals.
Proportion of females breeding and mate monopolizationAdult female curassows can breed every year (IBAMA 2004), but some may not do so for various reasons, and eggs can be lost to predation or accident (Lima et al. 2008). An annual breeding rate of 70% was therefore assumed for adult female curassows.
These values were derived from the CRAX Brasil breeding center experience, and we did not consider data obtained at REGUA or by Lima et al. (2008), since these observations were random and not determined by a systematic methodology.
CatastropheThe annual probability of a population of vertebrates experiencing a die-off of 50% or more is inversely related to generation length, and a particular population has a ~14% chance per generation of a severe die-off (Reed et al. 2003). Since red-billed curassows have a generation length of six years (5.73 in the VORTEX model), there is a 14% chance of catastrophic events occurring at REGUA every six years, or a 2.44% chance in any given year. Strong winds every September could produce such a catastrophe at REGUA, because nests can be destroyed.
HuntingDespite strong awareness campaigns and well-resourced active wardening, illegal hunting of red-billed curassows was recorded three times in neighboring areas of REGUA within the 25-month study period (Bernardo et al. 2011a). Therefore, we considered one pair hunted per year in all scenarios. In considering the “current population” scenario, we simulated scenarios where losses to hunting were reduced by 50% (that is, one pair hunted every two years) and by 100% (no hunting whatsoever occurring at the study site).
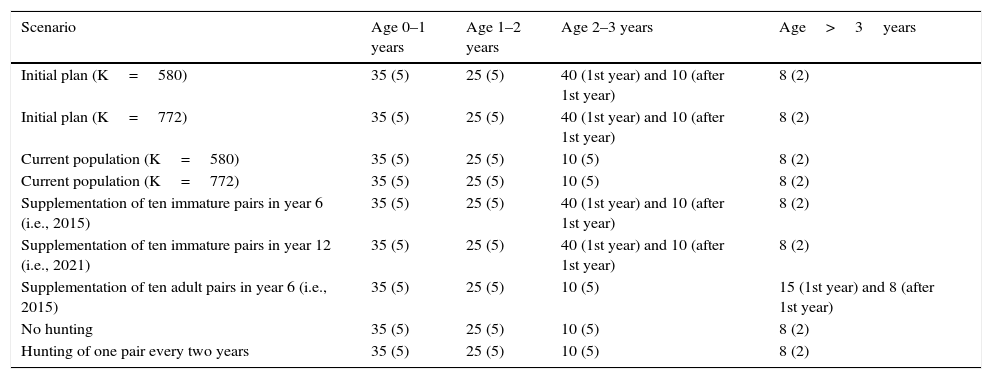
Mortality ratesAll birds released at REGUA were between 1 and 2 years old, and analysis showed an annual mortality probability of 25% (Bernardo et al. 2011b). This value was therefore used for immature birds (age class 1–2 years). Since we had no information on chick survival (i.e., in birds<1year old), chick mortality was assumed as 35% based on the generally high first-year mortality in wild populations (Begon et al. 2006) (Table 2). For birds aged 2–3 years, we assumed a mortality rate of 10%, reflecting a lower post-release vulnerability after the first year in the wild (when the 1- or 2-year-old released birds reached 2–3 years in the wild; Bernardo et al. 2011b; Table 2).
Mortality rate values (SD, standard deviation) for each age class, used in VORTEX to assess viability of the reintroduced red-billed curassow populations at REGUA, RJ, Brazil.
| Scenario | Age 0–1 years | Age 1–2 years | Age 2–3 years | Age>3years |
|---|---|---|---|---|
| Initial plan (K=580) | 35 (5) | 25 (5) | 40 (1st year) and 10 (after 1st year) | 8 (2) |
| Initial plan (K=772) | 35 (5) | 25 (5) | 40 (1st year) and 10 (after 1st year) | 8 (2) |
| Current population (K=580) | 35 (5) | 25 (5) | 10 (5) | 8 (2) |
| Current population (K=772) | 35 (5) | 25 (5) | 10 (5) | 8 (2) |
| Supplementation of ten immature pairs in year 6 (i.e., 2015) | 35 (5) | 25 (5) | 40 (1st year) and 10 (after 1st year) | 8 (2) |
| Supplementation of ten immature pairs in year 12 (i.e., 2021) | 35 (5) | 25 (5) | 40 (1st year) and 10 (after 1st year) | 8 (2) |
| Supplementation of ten adult pairs in year 6 (i.e., 2015) | 35 (5) | 25 (5) | 10 (5) | 15 (1st year) and 8 (after 1st year) |
| No hunting | 35 (5) | 25 (5) | 10 (5) | 8 (2) |
| Hunting of one pair every two years | 35 (5) | 25 (5) | 10 (5) | 8 (2) |
We assumed that 2-3-year-old birds used in supplementations would have a higher post-release vulnerability (40%) during the first year in the wild and lower mortality rate during the subsequent years (10%; Bernardo et al. 2011b), i.e., 10+((IS1=2)*30).
Because immature individuals released in larger groups experienced lower mortality than those released in pairs or alone (Bernardo et al. 2011b), we considered that immature birds supplemented in pairs would have a higher mortality (55%), i.e., 10+((IS1=2)*45) (Table 2).
For birds>3years old (that is, individuals that became adults in the wild), we assumed a low mortality rate (8%), assuming that after>2years in the wild, reintroduced individuals become sensitive to predation risks and are familiar with the local area (Table 2).
We did not build a scenario involving supplementation with adults because (1) we had no data on releases of adult red-billed curassows and (2) adults in captivity are currently used for reproduction and are not available for reintroduction.
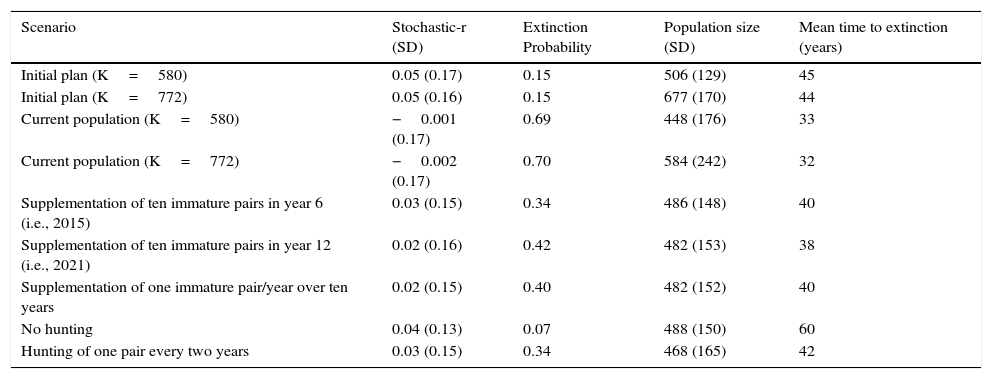
ResultsOur results indicate that the initial plan scenario (K=580) was likely to establish a viable population at REGUA, as shown by a probability of extinction below the threshold (PE=15%) and relatively high stochastic growth rate (0.05) (Table 3). The results are similar if carrying capacity is increased (K=772).
VORTEX simulation output for Red-billed Curassow populations at REGUA. Stochastic-r, stochastic population growth rate and SD, standard deviation.
| Scenario | Stochastic-r (SD) | Extinction Probability | Population size (SD) | Mean time to extinction (years) |
|---|---|---|---|---|
| Initial plan (K=580) | 0.05 (0.17) | 0.15 | 506 (129) | 45 |
| Initial plan (K=772) | 0.05 (0.16) | 0.15 | 677 (170) | 44 |
| Current population (K=580) | −0.001 (0.17) | 0.69 | 448 (176) | 33 |
| Current population (K=772) | −0.002 (0.17) | 0.70 | 584 (242) | 32 |
| Supplementation of ten immature pairs in year 6 (i.e., 2015) | 0.03 (0.15) | 0.34 | 486 (148) | 40 |
| Supplementation of ten immature pairs in year 12 (i.e., 2021) | 0.02 (0.16) | 0.42 | 482 (153) | 38 |
| Supplementation of one immature pair/year over ten years | 0.02 (0.15) | 0.40 | 482 (152) | 40 |
| No hunting | 0.04 (0.13) | 0.07 | 488 (150) | 60 |
| Hunting of one pair every two years | 0.03 (0.15) | 0.34 | 468 (165) | 42 |
According to VORTEX, the current population at REGUA was around 40 individuals in 2013 (Fig. 1). It is not viable over 100 years, as its probability of extinction is higher (69%) than the threshold (<40%). The stochastic growth rate is almost null (−0.001), which means that the population is highly vulnerable and subject to stochastic variations (Schaub et al. 2004) and could become extinct in 33 years (Table 3).
However, if the hunting pressure in the “current population” scenario is reduced by half, the probability of extinction reduces to 34% and the stochastic growth rate increases to 0.03. The probability of extinction significantly decreases if hunting is eliminated altogether (PE=7%), and this therefore represents the best mitigation scenario (Table 3).
Among the supplementation options, the release of ten immature pairs in year 6 (2015) results in the lowest probability of extinction (PE=34%; Table 3).
Our models suggest that the supplementation of a large group of immature birds in 2015 is better than releasing them in pairs over ten years, since the probability of extinction is lower (PE=34% vs. PE=40%; Table 3).
DiscussionThis study represents the first attempt to use PVA based on data collected at the release site for orienting strategic decisions after reintroducing a threatened bird species in South America. Quantitative assessment studies (e.g., PVA) for providing decisive insights into management are scarce and available in few regions of the world, such as New Zealand (Armstrong & Ewen 2001), Scotland (Green et al. 1996), and Alpine countries (Schaub et al. 2009).
These are successful projects and PVA indicated that the current reintroduced populations are self-sustainable in the long term. Thus, the authors recommend to stop releases and to focus on other conservation actions for the species (Green et al. 1996; Armstrong & Ewen 2001; Schaub et al. 2009). In the present study, we demonstrated the opposite: the current population of reintroduced red-billed curassows at REGUA is not viable in the long run, and supplementations are still needed in order to increase the viability of the current population.
We are aware that precise estimates of population viability are derived from longer time series, as alerted by Armstrong & Ewen (2001) and Schaub et al. (2009). However, we synthesized enough information about the species (e.g. reproduction and mortality rates) and developed the best possible model given the information available (Boyce 1992; Akçakaya & Sjögren-Gulve 2000). Thus, we believe that the results will arouse the interest of the natural resource managers involved in the conservation of the red-billed curassows to discuss the feasibility of the management decisions presented here.
REGUA population viabilityThe robustness of the initial plan was confirmed by our models, showing that the intended founder population size increased the chances of establishing a self-sustaining population (Fischer & Lindenmeyer 2000; Armstrong & Seddon 2007). The models also confirmed that the current population is too small for long-term viability. To increase the viability of this population, the best mitigation is the complete elimination of hunting, or at least its reduction by half. This threat helped cause previous local extinctions of the species, and although the deployment of seven rangers has greatly reduced the problem at REGUA (as recommended by IUCN/SSC 2013), some reintroduced birds were hunted outside REGUA’s boundaries (Bernardo et al. 2011b). Controlling hunting on lands adjacent to REGUA proves more important for population increase than supplementation (Table 3).
The supplementation of a large group (ten pairs) of immature birds in 2015 is the second best option to increase current population viability. We recommend the supplementation of immature rather than adult captive-bred birds, since the reintroduced immature birds that became adults at REGUA are experienced and less vulnerable to predators (survival of reintroduced adults is lower than immatures). Besides, adult captive-bred birds (of any species) used for supplementation are frequently inexperienced and have a reduced capacity to adapt or learn about predation risks (e.g. Asian Houbaras Chlamydotis macqueenii: Islam et al. 2010).
Our models suggest that releasing birds in larger groups (ten pairs) over one year is better than releasing them in pairs (smaller groups) over ten years. Moreover, releasing them as soon as possible (such as in 2015) guarantees a more viable reintroduced population at REGUA than if release is delayed (e.g., 2021).
These values will hopefully stimulate forward-planning by the only two breeding centers that can supply immature red-billed curassows for reintroduction in Brazil (Criadouro Científico e Cultural Poços de Caldas and CRAX Brasil, both in Minas Gerais, Brazil, and both private). Captive young redbilled curassows are currently scarce; this constrains the number of individuals that could be released at REGUA in 2015.
National sponsors/partnership: lack and needTo date, government participation in and funding for the reintroductions proposed in IBAMA (2004) has been inexistent. Current reintroductions have been paid entirely by private international institutions (the Brazilian-Japanese company CENIBRA and the British non-governmental organization Brazilian Atlantic Rainforest Trust). Funding mechanisms from environmental compensation schemes (action numbers 1.5 and 6.2 in IBAMA 2004) have regrettably not been explored. Such mechanisms can help sponsor breeding centers, genetic studies, transport of birds, and post-release monitoring programs.
We encourage the Brazilian government and private stakeholders to support the immediate supplementation of red-billed curassows at REGUA, since a positive long-term outcome there is still possible. Success or failure of the reintroduction at REGUA has significant implications for conservation in Brazil and beyond, because the reported experiences of a reintroduction program enable adjustments and improvements to be considered in future reintroduction plans (Sutherland et al. 2010). Increasing numbers of species are being held ex situ, given that these populations are vital to the long-term preservation of the species in question (Butchart et al. 2006). Examples from Brazil are the Alagoas Curassow Pauxi mitu and Spix’s Macaw Cyanopsitta spixii, both extinct in the wild and needing the best possible knowledge and experience of reintroduction theory and practice. We deem the red-billed curassow reintroduction at REGUA not only as a significant conservation initiative in itself but also a scientific model for future reintroductions of other species. The opportunity to continue the experiment and refine the model should not be missed.
We would like to thank Stephen Rumsey, Roberto Azeredo, James Pitt Simpson, Brian Creswell, Nicholas and Raquel Locke, Alan Martin, and Leigh Lock for their various contributions to the reintroduction program, which was sponsored by the Brazilian Atlantic Rainforest Trust. We also thank Dr. Kathy Traylor-Holzer for helping with the vortex model.
- Home
- All contents
- Publish your article
- About the journal
- Metrics