Several species of aquatic macrophytes have invaded ecosystems outside their ranges, producing a variety of impacts on native biota. In this study, we tested the role of the invasive macrophyte Hydrilla verticillata as a foraging habitat for small fish species. To achieve this goal, we assessed the feeding activity and diet composition of fish captured in patches of the invasive H. verticillata and of a similar native macrophyte (Egeria najas). Feeding activity did not differ significantly between H. verticillata and E. najas, indicating that foraging activity was not affected. However, differences in diet composition were significant in three out of five fish species and marginally significant in one species, suggesting that the invasive and native macrophytes provide different types of food resources for fish. Thus, although H. verticillata does not affect the foraging activity, it has the potential to affect the assemblages of small-sized fish through changes in the proportions of food resources.
© 2014 Associação Brasileira de Ciência Ecológica e Conservação. Published by Elsevier Editora Ltda.
Aquatic ecosystems have been colonized by large numbers of exotic species in recent decades (Jenkins 2003), including macrophytes. This process has also occurred in Brazilian freshwater ecosystems, including the Paraná River Basin (Agostinho et al. 2004). Studies of the effects of invasive macrophytes on aquatic assemblages have produced contradictory results. For example, invasive macrophytes have shown both negative (Colon-Gaud et al. 2004; Stiers et al. 2011) and positive (Strayer et al. 2003; Hogsden et al. 2007) effects on the density of specific invertebrate taxa.
The floodplain located in the upper stretch of the Paranáriver has suffered several anthropogenic changes, including water level regulation and increases in underwater light (Agostinho et al. 2004). These changes have facilitated the establishment of the submersed macrophyte Hydrilla verticillata (L.f.) Royle. H. verticillata is an aggressive colonizer, native to Asia and Australia (Cook & Lüönd 1982), which competes effectively for light, displacing native aquatic plants (Langeland 1996). This macrophyte was first recorded in the Paraná River in 2005, and it has rapidly invaded the main river and its lateral channels (Sousa 2011).
The macrophyte Egeria najas Planch. is the dominant submersed native species colonizing the Upper Parana River floodplain and it rarely co-occurs with H. verticillata (Sousa et al. 2010; Sousa 2011; Cunha et al. 2011). Both species have similar architectures and life forms (i.e., canopy forming) and thus, competitive interactions between them are expected (Sousa et al. 2010). Indeed, decreases in E. najas biomass following invasions by H. verticillata have been recorded in Paraná River habitats (Sousa 2011), and future scenarios that consider the replacement of E. najas by H. verticillata are a topic of concern.
In addition to its negative effects on native macrophytes, the spread of H. verticillata may have complex implications. For example, although it is morphologically similar to E. najas, H. verticillata may differ in terms of its associated organisms (e.g., microalgae and ostracods; Theel et al. 2008; Mormul et al. 2010a, b), which may serve as food resources for small sized-fish (Casatti et al. 2003; Pelicice & Agostinho 2006). Thus, since macroinvertebrates may be considered the primary link between plants and fish (Schultz & Dibble 2012), alterations of invertebrate assemblages following invasions by plants may produce cascade effects on fish assemblages.
In the Upper Paraná River floodplain, patches of native macrophytes (including E. najas) provide important habitats for small-sized fish (Dibble & Pelicice 2010; Cunha et al. 2011). Thus, given the high competitive ability of H. verticillata, other native macrophyte species may be excluded by this invasive plant, decreasing habitat heterogeneity and compromising the suitability of the habitat and the availability of food for fish.
Given the rapid spread of H. verticillata in the Upper Paraná basin, the invasion by this species may change other aquatic assemblages, influencing the availability of food to fish that use submersed macrophytes as feeding sites. Thus, in this investigation, we hypothesized that fish feeding activity and diet composition is affected by the invasive macrophyte H. verticillata. We predicted a change in the composition of food items eaten by small-sized fish, and that mean stomach fullness (SF) would be lower in H. verticillata than in E. najas. This prediction was based on the assumption that the two macrophytes differ in terms of organism composition (e.g., ostracods and epiphytic algae; Mormul et al. 2010a; Mormul et al. 2010b). To test our hypothesis, we compared the diet of fish species inhabiting monospecific patches of H. verticillata with the diet of fish inhabiting E. najas. The data for the fish inhabiting E. najas were considered to represent non-affected patches of habitat.
MethodsSampling was performed on August 6 and 7, 2009 in a lateral channel (“Cortado” channel) of the Upper Paraná River (22° 47’ 30” S, 53° 24’ 37” W; see Cunha et al. 2011 for more details) located within a National Protected Area. The channel is shallow (< 3m), is approximately 2km long×0.03-0.09km wide and has well-preserved riparian vegetation. In addition to our target species (E. najas and H. verticillata), this channel is colonized by other macrophytes, such as Eichhornia crassipes (Mart.) Solms, Eichhornia azurea (Sw.) Kunth, and Polygonum spp.
We selected three different sampling points (located a minimum of 500m apart) in which both E. najas and H. verticillata form monospecific patches with similar biomasses (which was confirmed by our further analyses – see Results) and separated by approximately 15 - 30m. Fish were sampled with transparent traps (Dibble & Pelicice 2010) within patches of each macrophyte. The traps were used in the top 30cm of the water column, because both species of macrophytes concentrate their biomass in this layer (Cunha et al. 2011). A pair of traps was installed at 7:00 in each patch and checked at 11:00, 15:00, and 19:00. Fish caught in each pair of traps and at different times of the day were pooled for analysis, given that no differences in feeding activity and diet composition were expected for the time period during which the samples were collected (Carniatto et al. 2012). All fish were anesthetized with eugenol before being fixed for further analyses.
In addition, haphazardly in each macrophyte patch, we measured the temperature, oxygen content (YSI digital meters), pH, and conductivity (Digimed DM-2P and DM-3P) in the sub-surface stratum of the water column. We quantified the illuminated percentage of water column with a Secchi disk near the patches. Plant biomass was also collected in a volume of 0.5×0.5×0.3m at the top of the water column and dried in an oven at approximately 80°C to a constant weight to test for differences in physical structure of the habitat between species of macrophytes.
We used 420 individuals belonging to five species: Astyanax altiparanae (Garutti & Britski, 2000) (standard length [SL] range=20.2 - 46.1mm); Moenkhausia bonita (Benine, Castro & Sabino, 2004) (SL=9.0 - 37.5mm); Hyphessobrycon eques (Steindachner, 1882; SL=15.4 - 23.9mm); Pamphorichthys sp. (SL=10.3 - 24.4mm); and Serrapinnus notomelas (Eigenmann, 1915; SL=12.1 - 33.6mm). Stomachs were visually assessed for the degree of SF using the following numerical scale: 0=empty stomach; 1=up to 25% SF; 2=25% to75% SF ; 3≥75% SF. We assessed feeding activity using the value of mean SF: mSF=(N0×0)+(N1×1)+(N2×2)+(N3×3)/N, where N0, N1, N2, and N3 are the number of stomachs with SF values of 0, 1, 2, and 3, respectively, and N is the number of individuals (Santos 1978). For diet analysis, food items were identified and quantified with the volumetric method (Hyslop 1980).
Differences in the physical and chemical characteristics of the habitat and in macrophyte biomass between E. najas and H. verticillata were tested with a one-way analysis of variance (ANOVA) using Statistica 7.0 (STATSOFT 2005). To test whether the feeding activity differed between H. verticillata and E. najas, we applied a Mann-Whitney non-parametric test to the mSF values. We used this test because our data did not have a normal distribution. Pamphorichthys sp. was not used in this analysis because this species does not have a clearly defined stomach. Mann-Whitney analyses were performed in Statistica 7.0 (STATSOFT 2005). To assess whether the fish diet composition differed between the two macrophytes, we employed a permutational multivariate analyses of variance (PERMANOVA), using the Bray-Curtis dissimilarity of the logtransformed data matrix containing the volumes of food items; type III sums of squares were used to account for unbalanced statistical design (Quinn & Keough 2002). We used 9,999 permutations to assess the significance of the F statistic derived from the PERMANOVA. Multivariate analyses were performed in PRIMER 6.1.13 and in the PERMANOVA+1.0.3 add-on (Anderson et al. 2008; Clarke & Gorley 2001).
ResultsThe macrophyte patches varied from approximately 4 to 15m2. Patches of H. verticillata and E. najas did not differ in terms of temperature (H. verticillata: 22.3±1.2, E. najas: 22.2±1.1°C), oxygen content (H. verticillata: 7.8±1.2, E. najas: 7.7±2.3mgL-1), pH (H. verticillata: 6.8±0.4, E. najas: 6.6±0.3), or conductivity (H. verticillata: 61.1±3.6, E. najas: 59.2±3.6 μS cm–1) (all p-values>0.05). The water column was completely illuminated at all sampling points (Secchi depth always reached the sediment), and the mean macrophyte biomass did not differ significantly (p=0.55) between H. verticillata (281.78±57.30 gDW m-3) and E. najas (312.09±57.77 gDW m-3). In conjunction, these results indicate that the patches of both macrophytes provide similar habitats in terms of physico-chemistry and physical structure (as indicated by plant biomass) for fish.
Feeding activity was high for the four species analyzed (mSF>2.1), indicating that the fish used both macrophytes as feeding sites. The differences in mSF between H. verticillata and E. najas were not significant (Table 1).
Mean stomach fullness (mSF) of the fish species caught in patches of Hydrilla verticillata (H) and Egeria najas (E) and values of the Mann-Whitney test. The numbers of stomachs analyzed are in parentheses.
| msF | Mann-Whitney | |||
|---|---|---|---|---|
| H | E | U | P | |
| Astyanax altiparanae | 2.36 | 3.00 | 1.5 | 0.191 |
| (7) | (4) | |||
| Moenkhausia bonita | 2.66 | 2.36 | 1.0 | 0.121 |
| (67) | (118) | |||
| Hyphessobrycon eques | 2.32 | 2.11 | 3.5 | 0.650 |
| (10) | (44) | |||
| Serrapinnus notomelas | 2.41 | 2.56 | 2.0 | 0.275 |
| (49) | (114) | |||
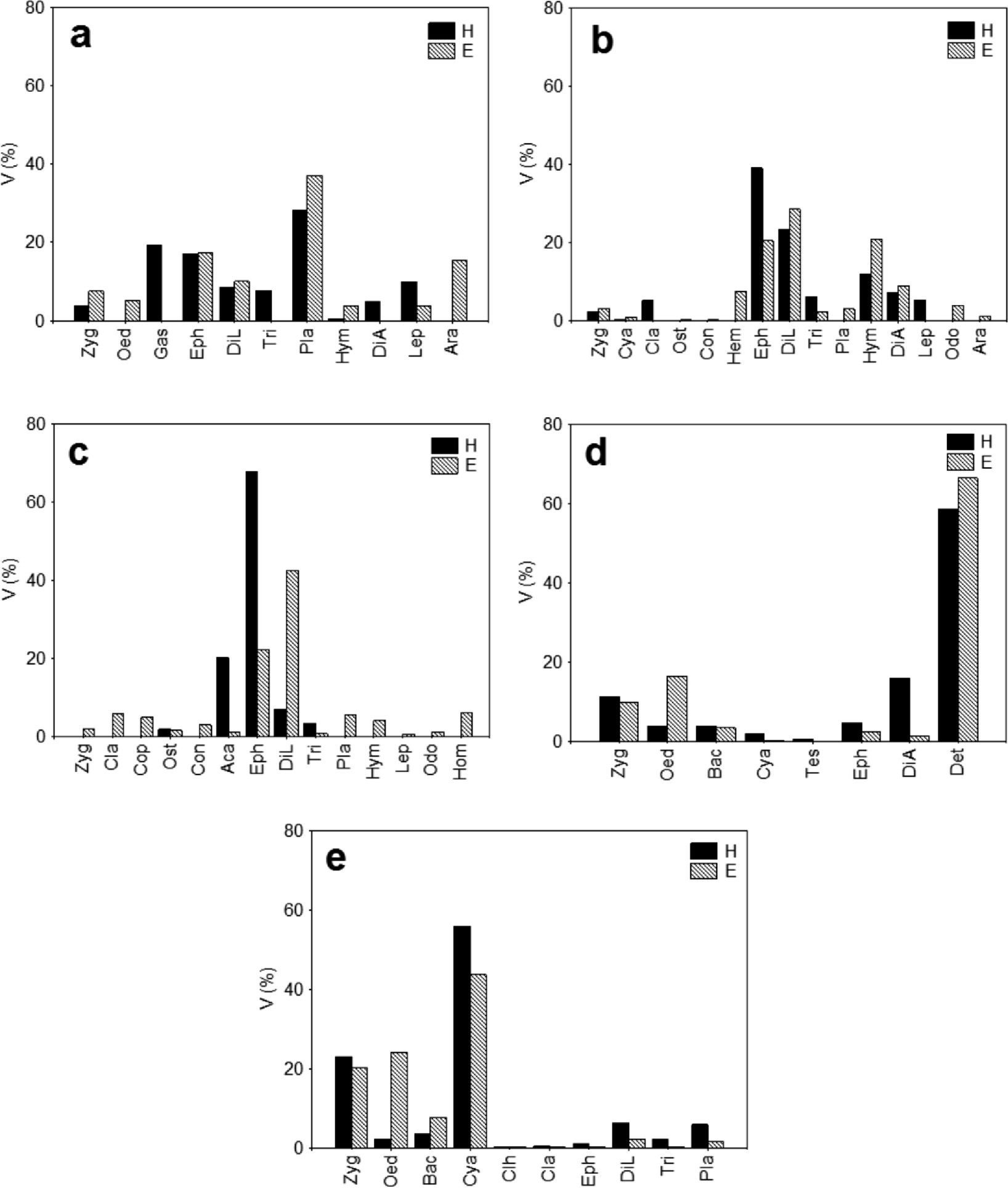
With the exception of Pamphorichthys sp., the diet of the fish caught inside patches of H. verticillata differed from those caught inside patches of E. najas, although the difference was marginally significant for M. bonita (p=0.06; Table 2). Higher plants and Ephemeroptera dominated the diet of Astyanax altiparanae in both macrophytes; however, Gastropoda, Trichoptera, and Diptera (adult) were exclusive in H. verticillata, and Diptera (larvae) and Aranae, in E. najas (Fig. 1a; Table S1, supplementary material online). The main items consumed by Moenkhausia bonita caught in H. verticillata were Ephemeroptera and Diptera (larvae), whereas in E. najas, the most abundant food items were Diptera (larvae), Ephemeroptera, and Hymenoptera (Fig. 1b; Table S1, supplementary material online). There were also differences between the macrophytes in the consumption of Cladocera, Lepidoptera, Hemiptera, and terrestrial Diptera.
Results of a permutational multivariate analysis of variance (PERMANOVA) applied to data on the diets of fish species caught in patches of Hydrilla verticillata and Egeria najas.
| Species | PERMANOVA |
|---|---|
| A. altiparanae | pseudo-F1,9=1.96; p=0.03 |
| M. bonita | pseudo-F1,74=1.87; p=0.06 |
| H. eques | pseudo-F1,50=4.12; p<0.001 |
| Pamphorichthys sp. | pseudo-F1,23=0.96; p=0.44 |
| S. notomelas | pseudo-F1,154=8.81; p<0.001 |
Percentage of volume of food items composing the diet of fish caught in patches of Hydrilla verticillata (H) and Egeria najas (E). a=Astyanax altiparanae; b=Moenkhausia bonita; c=Hyphessobrycon eques; d=Pamphorichthys sp.; e=Serrapinus notomelas. Zyg=Zygnemaphyceae; Oed=Oedogoniophyceae; Bac=Bacillarophyceae; Cya=Cyanophyceae; Clh=Chlorophyceae; Tes=Testacea; Gas=Gastropoda; Cla=Cladocera; Cop=Copepoda; Ost=Ostracoda; Con=Conchostraca; Aca=Acarina; Hem=Hemiptera; Eph=Ephemeroptera; DiL=Diptera (larvae); Tri=Trichoptera; Det=Detritus/sediment; Pla=Higher plants; Hym=Hymenoptera; DiA=Diptera (Adult); Lep=Lepidoptera; Odo=Odonata; Hom=Homoptera; Ara=Araneae.
The diet of Hyphessobrycon eques consisted primarily of Ephemeroptera in H. verticillata and of Diptera (larvae) and Ephemeroptera in E. najas (Fig. 1c; Table S1, supplementary material online). Pamphorichthys sp. primarily consumed detritus in both macrophytes, and its diet did not differ between the macrophyte species (Fig. 1d; Table S1, supplementary material online). Serrapinnus notomelas primarily ate algae (mainly Cyanophyceae) in both H. verticillata and E. najas; however, in certain instances, such as the algae Oedogoniophyceae, Diptera (larvae) and higher plants were consumed in different proportions between macrophytes (Fig. 1e; Table S1, supplementary material online).
DiscussionThe information regarding feeding activity obtained by this study indicates that macrophyte species did not influence the foraging activity of fish, suggesting that H. verticillata provides food resources that are quantitatively comparable to those furnished by the native E. najas. The lack of differences in fish feeding activity between invasive and native macrophytes might have occurred because both plants have similar physical complexity and occupy the same stratum of the water column (Cunha et al. 2011). Because H. verticillata and E. najas have similar morphology, they most likely support similar densities of invertebrates, resulting in similar amounts of food to fish. Indeed, previous investigations in the Upper Parana floodplain showed that the densities of some invertebrate groups did not differ between H. verticillata and E. najas (Mormul et al. 2010a). A lack of differences in invertebrate abundance, biomass, and species richness has also been found by a comparison of monospecific patches of H. verticillata with multispecific patches of macrophytes in ponds in the southern USA (Theel et al. 2008). From such evidence, together with our findings, we infer that H. verticillata provides a suitable amount of food resources to the small-sized fish that inhabit its patches. However, this interpretation should be considered in the light of our sampling strategy, since we chose patches of macrophytes where H. verticillata and E. najas attained similar biomasses. In this respect, it is difficult to predict whether differences in feeding activity might appear if the biomass of H. verticillata is greater than that of E. najas, as observed in other habitats (Sousa et al. 2010). Thus, this scenario involving biomass differences remains to be tested before any general inferences can be drawn.
The most frequently consumed items are organisms associated with macrophytes (e.g., epiphytic Oedogoniophyceae and Cyanophyceae, Acarina, Ephemeroptera, and Diptera larvae). The use of organisms associated with macrophytes, together with the results of feeding activity, indicate that the small-sized fish use patches of both species of macrophytes as feeding sites. Although fish could move from one macrophyte patch to the other in the field, our conclusion that fish use both plants as feeding sites is re-enforced by experiments showing that fish exposed to both plants in isolation had high and similar feeding activity (N. Carniatto, unpublished).
Despite the similarities in feeding activity that we found, diet composition differed in three species and marginally differed in one species of fish between H. verticillata and E. najas patches, indicating that the invasive macrophyte does not provide the same proportion of food items as the native plant. In addition, this pattern appears to be even stronger for species that consume aquatic invertebrates. Because both macrophytes have the same architecture, we infer that differences in invertebrate assemblages may be associated with foliar texture, growth and senescence rates, and allelopathic compounds, which may affect invertebrate colonization (Taniguchi et al. 2003; Vieira et al. 2007).
Aquatic invertebrates (primarily insects) were the main items consumed by A. altiparanae, M. bonita, and H. eques in both macrophytes sampled. Differences in the consumption amount among these items, together with others that were less abundant, produced the observed differences in the composition of the diet. These findings should mirror the real differences between the types of available items provided by each species of macrophyte. For example, Ephemeroptera and Trichoptera were more important in the diet of the fish colonizing H. verticillata, whereas aquatic Diptera were more important in the fish colonizing E. najas. Several items with less predominance in the diet also differed: Gastropoda were found only in the fish colonizing H. verticillata, whereas Copepoda, Conchostraca, Hemiptera, and Homoptera were found only in the fish colonizing E. najas. These results indicate that H. verticillata provides invertebrates other than those found in E. najas. Indeed, in a manipulative experiment in the Upper Paraná River, these two plants differed in terms of ostracod assemblages (Mormul et al. 2010a), corroborating our findings for the fish stomachs analyzed in the current study.
Microalgae were the dominant item in the diet of S. notomelas in both macrophytes; however, there was a higher consumption of Oedogoniophyceae in E. najas. Given the significant results for the diet composition for this species, these results may also reflect differences in the algal species available in these two macrophytes, as the two plant species show distinct patterns of colonization by epiphytic algae (Mormul et al. 2010b). Pamphorichthys sp., which was the sole species of fish that clearly showed no differences in diet composition between the macrophyte species, has a restricted diet consisting of detritus and sediment. Detritivory is one of the most specialized feeding habits in fish (Gerking 1994); what may explain the lack of difference in the diet between the habitats used by Pamphorichthys sp.
In summary, our results indicate that H. verticillata did not influence the foraging activity of small-sized fish, but it affected their diet composition. We suggest that these differences are results of the availability of food items, such as invertebrates and microalgae, which most likely tend to colonize each species of macrophyte in different ways. Whether the differences in diet composition found in this work are sufficient to influence the composition of assemblages of small-sized fish and to produce cascade effects are questions for further investigation. Although similar foraging activity was found in the two plant species, we continue to view H. verticillata with concern, since fish movement and feeding are limited in sites with high plant densities (Dibble et al. 1996; Harrel & Dibble 2001) and H. verticillata has the potential to grow much more rapidly (Bianchini Jr. et al. 2010) and achieve a higher biomass than native macrophytes (Sousa et al. 2010; Sousa 2011). We suggest that future investigations should test the effects of a gradient of H. verticillata biomass on fish feeding activity and diet composition.
AcknowledgmentsWe thank RR Ota for fish identification, and we thank JC Oliveira and CF de Souza for helping with the stomach contents. N Carniatto acknowledges the Brazilian Council of Research (CNPq) for providing grants, and SM Thomaz also thanks CNPq for continuous funding through a Research Productivity Grant. This study was supported by CAPES, a Brazilian organization focused on the development of human resources.
Supplementary materialSupplementary material associated with this article can be found, in the online version, at www.naturezaeconservacao.com.br.