Different biological communities may exhibit similar spatial and/or temporal distributional patterns, a property termed community concordance. This study was conducted in a tropical irrigation system (Araguaia River floodplain) and aimed to quantify concordance levels between three aquatic communities (zooplankton, benthic macroinvertebrates, and aquatic macrophytes), and between these communities and a set of environmental predictors. To accomplish these goals, we used ordination techniques and Procrustean analysis. There were no relationships between the communities, and only zooplankton community patterns were significantly correlated with environmental predictors. These results indicate that biological surrogacy can be a flawed approach at small spatial scales and highlight the importance of the zooplankton community as a reliable ecological indicator in this type of system.
© 2014 Associação Brasileira de Ciência Ecológica e Conservação. Published by Elsevier Editora Ltda.
Ecological studies recurrently search for patterns in the spatiotemporal distribution of organisms and attempt to explain these patterns according to environmental gradients, biotic interactions, and dispersal processes (Bowman et al. 2008). However, a particular biological community can also be used to predict the distribution of the community of interest (i.e., the response “species x samples” data matrix). It may be asked, for instance, whether different groups of aquatic organisms generate patterns of classification or ordination of floodplain lakes in a similar way or, simply, whether they are concordant. If they are concordant, then we can try to identify the mechanism behind this pattern. Recent studies have suggested that biotic interactions (competition, predation, or facilitation) are the most likely mechanisms underlying concordance (see Johnson & Hering 2010). Moreover, concordant responses are also expected to arise from similar responses to environmental gradients (Bini et al. 2007; Rooney & Bayley 2012). As an important implication, strong levels of concordance may indicate that only one of the concordant groups could be used in biological monitoring programs (Sanchez-Fernandez et al. 2006), reducing costs and sampling processing time.
Aquatic macrophytes play an important role in the formation of environmental gradients. For instance, macrophytes are an important source of organic matter for benthic organisms and probably are the main substratum for periphyton in Neotropical lakes (Padial et al. 2012). Also, these plants can influence different communities by decreasing water velocity, increasing flood supply, and providing refuges (Declerck et al. 2007; Thomaz et al. 2008). Drastic changes in ecosystem properties (e.g., water transparency and nutrient concentrations) can be caused by changes in the composition of aquatic plants (Scheffer 2004). Thus, directly or indirectly, changes in the species composition of aquatic plants are likely related to changes in the structure of other aquatic communities.
Floodplain systems around the world have been intensively modified by different human activities, including agriculture-related impacts (e.g., water diversion and contamination; Lemly et al. 2000). However, agroecosystems (e.g., irrigated rice fields) can still contribute to regional biodiversity (Maltchik et al. 2011). Thus, the use of surrogate groups, after validation, may be a cost-effective strategy to monitor the status and attest the ecological importance of these systems. In the present study, we tested for patterns of community concordance between zooplankton, benthic macroinvertebrate, and aquatic macrophyte communities inhabiting a rice irrigation system in the Araguaia River floodplain of Brazil. We also tested the relationship between these communities and limnological predictors. We predict strong patterns of community concordance, considering the strong structuring role of aquatic macrophytes in shallow aquatic environments.
Material and methodsStudy areaThis study was conducted at the Sistema de Irrigação Luís Alves do Araguaia. This is a rice (Oryza sativa L.) irrigation system located in the Araguaia River floodplain (50°32’ W and 13°12’ S, São Miguel do Araguaia City), Goiás State, Brazil, with area of approximately 21,427,800m2. Data were gathered during March of 2005 at six sampling sites: (1) Lago de Luís Alves (LL), a lake that is used as water source for the whole system; (2) Rio Verde (RV), a river that receives the irrigation effluents; (3) adduction channel (AC); (4) drainage channel (DC); (5) secondary channel (SC); and (6) Lago do Brito (LB), a lake within the floodplain but outside the irrigation system (Fig. S1, supplementary material online).
Environmental variablesThe following environmental variables were measured with a digital probe (WD-35642-60 model): pH, dissolved oxygen, and conductivity. Water samples were analyzed for biochemical oxygen demand (BOD), turbidity, iron, total Kjeldahl nitrogen, potassium, manganese, and sodium concentrations following the methods described by APHA (2005).
Biological dataZooplankton samples were taken by pumping 1,000L of water through a 68μm plankton net. Samples were fixed immediately with 5% buffered formalin. With the use of a Sedgwick-Rafter chamber and an optic microscope, we counted five subsamples (2mL each) taken with a Stempel pipette from the concentrated samples (100mL). Zooplankton density was then measured as individuals per m3. Zoobenthic community was sampled by taking two sub-samples at each sample site with a Petersen grab (0.0252m2). Samples were kept in plastic bags with 4% formaldehyde. In the laboratory, the material was washed through a series of sieves with different mesh sizes (0.15mm to 50mm), and all individuals were counted and identified with the aid of a stereoscopic microscope. Macrophyte surveys were conducted in the sampling sites and in areas downstream and upstream of these sampling sites until the detection of all species. Emergent, floating, or leaf-floating macrophytes were collected manually, whereas submerged individuals were searched with a rake.
Data analysisDetrended correspondence analysis (DCA; Hill & Gauch 1980) was used to ordinate sites according to each aquatic community (zooplankton, zoobenthos, and aquatic macrophytes). To test the effect of numerical resolution on patterns of community concordance, we used density and presence/absence datasets. A principal component analysis (PCA; Legendre & Legendre 1998) from a correlation matrix was performed to ordinate sites according to the abiotic variables. Except for pH and incidence data (in the case of aquatic macrophytes), all variables were log-transformed prior to analyses.
Procrustes analyses (Jackson 1995) were undertaken based on the scores of the first two DCA and PCA axes to quantify the levels of concordance between the communities and their relationships with the environmental variables. Procrustes analysis provides a measure of lack of fit or lack of concordance, which is the sum of the squared deviations between the corresponding ordinations (m2). After, this measure can be converted to a correlation statistic r (the square-root of 1-m2), varying from 0 (no concordance) to 1 (total concordance). Its statistical significance (PROTEST) was assessed by 720 Monte Carlo randomizations (Jackson 1995). Significant r-values would then indicate that pairs of communities are concordant or that there are associations between community structure and environmental data.
ResultsMost environmental variables showed high levels of variation, as observed by the coefficients of variation (Table 1). The first two principal components explained 85.2% of the total variation in the environmental dataset. RV stands out as the most dissimilar sampling site, showing unique environmental conditions (Fig. 1, Table 1). The highest values for the variables negatively correlated with the first principal component (turbidity, conductivity, potassium, sodium, iron, and manganese contents) were registered in DC and SC (whereas the lowest values were found at RV). The sampling point LB showed the highest values of pH and BOD (which were positively correlated with the second axis), whereas SC, AC, and RV presented the highest nitrogen concentrations (Fig. 1, Table 1).
Summary statistics of the environmental data and Pearson’s correlation coefficients between environmental variables and principal component analysis (PCA) axes scores (values≥0.6 are in bold).
| Environmental variables | Mean | Minimum | Maximum | CV | Pearson correlation (r) | |
|---|---|---|---|---|---|---|
| 1st Axis | 2nd Axis | |||||
| Turbidity(NTU) | 24.2 | 3.4 | 45.9 | 64.0 | -0.99 | -0.11 |
| Potassium (mg/L) | 1.5 | 0.2 | 3.5 | 71.9 | -0.99 | 0.12 |
| Sodium (mg/L) | 1.6 | 0.5 | 2.4 | 42.4 | -0.97 | 0.03 |
| Conductivity (μS/cm) | 30.7 | 12.0 | 46.0 | 38.2 | -0.97 | 0.20 |
| Iron (mg/L) | 1.4 | 0.2 | 1.7 | 42.6 | -0.92 | 0.10 |
| Manganese (mg/L) | 0.1 | 0.0 | 0.1 | 41.9 | -0.92 | 0.00 |
| Nitrogen (mg/L) | 8.3 | 7.8 | 9.3 | 7.7 | -0.57 | -0.76 |
| Oxygen saturation (%) | 59.8 | 38.0 | 80.1 | 27.9 | -0.48 | -0.59 |
| Biochemical oxygen demand (mg/L) | 0.7 | 0.1 | 1.9 | 116.0 | -0.57 | 0.64 |
| pH | 6.7 | 6.2 | 7.6 | 7.2 | -0.03 | 0.84 |
CV=coefficient of variation (%).
Detrended correspondence analysis (DCA) and principal component analysis (PCA) results. LL=Lago de Luiz Alves; AC=adduction channels; DC=drainage channel; LB=Lago do Brito; RV=Rio Verde; SC=secondary channel; K=potassium; EC=conductivity; Turb=turbidity; Na=sodium; Fe=iron; Mn=manganese; BOD=biochemical oxygen demand; N=total Kjeldahl nitrogen.
One hundred and three (103) taxa were identified: 62 for zooplankton, 17 for zoobenthos, and 24 aquatic macrophytes. For zooplankton, the highest species richness was found in sampling sites LL and AC, whereas the lowest value was observed at RV. The lentic environments (LL and LB) showed the highest density values. For zoobenthos, both density and richness reached maximum values in lotic habitats (DC and RV; Fig. S2, supplementary material online). Aquatic macrophytes richness peaked at LL, LB, and RV (Fig. S2, supplementary material online).
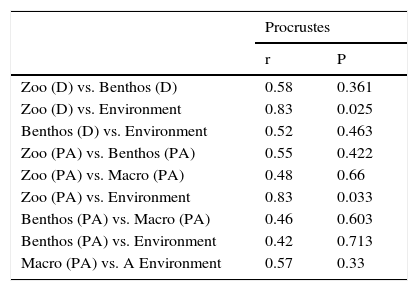
The results from DCA performed for each taxonomic group and different numeric resolutions (i.e., density or presence/absence data) showed variable ordination patterns (Fig. 1). However, it can be observed that RV stands out as the most dissimilar sampling point in most of the ordination plots (Fig. 1), except for the analysis performed with zoobenthos density data. The Procrustes tests detected a significant pattern of concordance only between zooplankton community structure and environmental variables (Table 2).
Results from Procrustes analyses evaluating the relationships between biological communities and between communities and environmental variables. The following data was used in the comparisons: zooplankton (Zoo), Zoobenthos (Benthos), Macrophytes (Macro) and the environmental variables (Environment).
| Procrustes | ||
|---|---|---|
| r | P | |
| Zoo (D) vs. Benthos (D) | 0.58 | 0.361 |
| Zoo (D) vs. Environment | 0.83 | 0.025 |
| Benthos (D) vs. Environment | 0.52 | 0.463 |
| Zoo (PA) vs. Benthos (PA) | 0.55 | 0.422 |
| Zoo (PA) vs. Macro (PA) | 0.48 | 0.66 |
| Zoo (PA) vs. Environment | 0.83 | 0.033 |
| Benthos (PA) vs. Macro (PA) | 0.46 | 0.603 |
| Benthos (PA) vs. Environment | 0.42 | 0.713 |
| Macro (PA) vs. A Environment | 0.57 | 0.33 |
D=density data; PA=species presence/absence data.
Except for the strong association between zooplankton community structure and environmental characteristics, no other pattern of concordance was found, either between the communities or between the communities and the environmental variables. An important initial discussion related with the search for surrogates groups, which goes beyond the analysis of p-values, has to do with the analysis of effect sizes. In this context, we found intermediated level of concordance (r-values ranging from 0.42 to 0.83). A review conducted by Heino (2010), for instance, indicated similar levels of concordance, with r-values generally lower than 0.71 (or m2>0.5). Thus, independently of the significance levels, we would suggest the use of surrogates only in instances in which large concordance levels are found.
The lack of concordance would suggest that either (i) each taxonomic group studied here have a unique response to environmental gradients (not investigated by us) or (ii) the spatial extent of our study area was too small to contain wide environmental gradients that could produce patterns of concordance. In a study aiming to test the relationships among macroinvertebrates, bryophytes, and fishes, Paavola et al. (2006) found that patterns of concordance were much less evident at small scales (as in a single river) than in a whole ecoregion. It is expected, therefore, that the responses of taxonomic groups to environmental gradients are similar at regional scales (Heino 2001) and different in local scales that encompass low environmental variability (Paavola et al. 2006; Bini et al. 2007). We argue, however, that low environmental variability is not a suitable explanation to the absence of significant relationships, as many variables known to be important in structuring aquatic communities (e.g., turbidity, oxygen saturation, and conductivity) were found to have high coefficients of variation. In the same vein, Dolph et al. (2011) demonstrated that significant patterns of community concordance were found at different spatial scales and that community concordance degrees were, in some cases, even stronger at small spatial scales (catchments) than at larger spatial scales (ecoregions).
A number of studies have demonstrated a strong role of aquatic macrophytes on different aquatic communities (Scheffer 2004 and references therein), even when their abundances are low (Gasith & Hoyer 1998). Therefore, the lack of concordance between aquatic plants and other communities is surprising. Given the paucity of studies on community concordance encompassing this study specifically, it is premature to speculate further on the importance of aquatic plants in generating patterns of community concordance.
Compared to other groups, the zooplankton community is expected to have the shortest life cycle and, therefore, to display stronger responses to environmental gradients (Allen et al. 1999). Indeed, at the small spatial scale investigated by us, it was the only group significantly correlated with the environmental variables. This result indicates the importance of the zooplankton community in biomonitoring programs. Conversely, as indicated above, at least in the irrigation system studied here, it cannot be considered as a surrogate for other biological groups.
Biomonitoring programs can benefit from the use of surrogate groups to predict the structure of other biological groups (Landeiro et al. 2012) and also indicate human disturbances in wetlands (Rooney & Bayley 2012). Stevens et al. (2007), for instance, studied the performance of beavers as surrogates for the conservation of stream amphibians, whereas Paszkowski & Tonn (2006) found highly concordant patterns between trophic guilds of birds, suggesting that monitoring could be conducted with only one of them. Other studies, however, demonstrated that the use of surrogates is not reliable in every circumstance (Bini et al. 2007; Lopes et al. 2011). Thus far, the weight of evidence suggests that the use of surrogates as a strategy for minimizing biomonitoring costs is unreliable (Heino 2010). This implies that the results obtained with a particular biological group should not be extrapolated to other, unanalyzed, groups. Therefore, it is important to highlight that the reliability of the surrogacy approach should be tested rather than assumed.
AcknowledgmentsThis work was partially funded by the Decanato de Pesquisa e Pós-Graduação da Universidade de Brasília (DPP/UnB) and the Fundação de Empreendimentos Científicos e Tecnológicos (Finatec). CNPq and CAPES have continuously supported our research group in aquatic ecology.
Supplementary materialSupplementary material associated with this article can be found, in the online version, at www.naturezaeconservacao.com.br.