We investigated the richness, density, and composition of woody-tree species, and which traits of colonizing species are selected in commercial stands of Pinus elliottii and Araucaria angustifolia in southern Brazil. Analyses of variance with permutation tests were performed to assess differences among stand types in relation to density and rarefied richness. Multivariate analyses of variance with permutation tests were performed to compare stand types in relation to species composition and reproductive traits. Native forests presented higher stem density when compared to the plantations, while species richness did not vary among stand types. Species composition differed between the native forest and the plantations. Species' reproductive traits differed between the two types of plantations. The higher frequency of zoochorous diaspores in P. elliottii plantations when compared to the other stand types suggests the importance of the fauna in creating and sustaining the understory structure in this type of plantation. Plantations show a high potential as colonization sites by native woody species, despite their structural differences in relation to native forests. If properly managed, plantations might be catalysts for the recovery of degraded areas.
© 2014 Associação Brasileira de Ciência Ecológica e Conservação. Published by Elsevier Editora Ltda.
Human alteration of tropical-forest habitats is one of the main causes of biodiversity loss at local and regional scales. However, monocultures of exotic species can be used as catalysts for the recovery of degraded areas (Senbeta et al. 2002; Barbosa et al. 2009). Dispersal of propagules is fundamental to biodiversity restoration, and can be potentially accelerated by forestry practices on degraded land (Wunderle 1997). The disturbance associated with monocultures of exotic trees can affect the structure of the vegetation, which may benefit from the presence of species with particular dispersal traits (Decocq et al. 2004).
The seed dispersal syndrome (Barbosa et al. 2009) and the physical characteristics of the diaspore (Neri et al. 2005), among other traits, can favor the colonization of particular tree species in commercial plantations. The enhancement of habitat structural complexity is associated with an increase in plant diversity, which by its turn might attract different propagule dispersers. The abundance, diversity, and food preferences of frugivorous animals can affect the forest structure (Clark et al. 2001).
The Atlantic Forest is one of the largest humid tropical forests of the Americas. It once covered approximately 150 million hectares of highly heterogeneous environments (Ribeiro et al. 2009). The Mixed Ombrophilous Forest (MOF) is situated in the southern part of the Atlantic Forest, and is characterized by the presence of the Paraná pine (Araucaria angustifolia). Over the last centuries, this forest has been largely devastated, and presently occupies approximately 12% of its original extent (Ribeiro et al. 2009). Pinus elliottii has replaced A. angustifolia in the timber industry by the 1950s and since then the landscape has been changed by the insertion of the exotic tree plantations. Thus, it is important to evaluate the dispersal traits of tree species colonizing plantations for successful management and restoration practices.
In the present study, our aim was to evaluate the colonization of different kinds of plantation stands by tree species in relation to diversity patterns and plant dispersal traits. We examined three hypotheses: 1. Commercial plantation stands with exotic trees show lower species richness and density of native trees than stands with native tree plantations and native forests; 2. tree species occurring in the plantations represent a subset of the species present in the native forests; and 3. native forests present more variable dispersal traits than plantation stands.
Material and methodsStudy areaThe study was conducted in stands of commercial plantations of P. elliottii and A. angustifolia, both with presence of understory, as well as in areas of native forests in a commercial tree farm located in the municipality of Campo Belo do Sul (27°59' S and 50°53' W), southern Brazil (1,000m asl). The area has 17,000ha; half of it is used for Pinus plantations and only 500ha are used for the Araucaria plantations. The climate is typical of the southern Brazilian highlands, with cool summers, no dry season, and frequent severe frosts (Köppen Cfb classification). The mean annual temperature is 16° C.
The characteristic feature of the region is the Araucaria forests, which occur mainly between 400 and 1,000m asl along watercourses, valleys, hillsides, and grasslands on the plateau (IBGE 1992). The presence of A. angustifolia determines the vegetation physiognomy; these trees occur in continuous forest habitats, as well as in patches embedded in the plateau grasslands. The forest on the hillsides and valleys of the study area can be considered a disturbed forest, but with a high level of biotic integrity, since it was only selectively logged a few decades ago. At the time of the study, the Pinus elliottii plantation was 27 years old and the A. angustifolia plantation areas were circa 30 years old. Both plantations were surrounded by native forests. Thus, any species colonizing the plantations were likely dispersed from a neighbor native forest.
Sampling designFour areas were located within each of three different vegetation types: native forest (NF), P. elliottii plantations (PP), and A. angustifolia plantations (AP). The mean distance between the areas was 8km, and between each area in the vegetation type, 2km. In each sampling site, six transects (at least 100m from the edge) were delimited 100m distant from each other. Perpendicular 50m-long lines were established from each of the six marked transects. Within each line, five secondary points (10m from each other) were marked. In each secondary point, a point-centered quarter (Cottam & Curtis 1956) was established, and the closest individual tree with a diameter at breast height (DBH) ≥ 47mm was sampled in each of the four quadrants, totaling 150 points for each vegetation type. Subsequently, the individuals were identified to species level and the density of these was calculated. Field data was obtained from January to April of 2009. In the plantations, individuals that were conspecific with the planted trees were disregarded in the analysis. This precaution was needed because our main goal was to analyze the arrival of propagules from outside the stands.
Plants were characterized by traits related to dispersal and attractiveness for animals: dispersal syndrome (zoochorous or not), color (brown, red, yellow, black, orange, purple, green, or white), type (legume, drupe, pine, capsule, cypsela, berry, achene, samara, craspedium, syncarp, follicle), and size of diaspore, based on the literature and field data (Table S1, supplementary material online). We then computed the frequency of occurrence (for categorical variables) or the community weighted mean (for diaspore size) of each trait in each area, which rendered a multivariate trait matrix describing each area by frequency/mean values of traits (Pillar et al. 2009).
Statistical analysesRichness values computed in each sampling site were rarefied to remove the effect of sample size according to the individuals that were disregarded (Gotelli & Colwell 2001) using the software PAST (Hammer et al. 2001). Next, the rarefied richness values computed for the different stand types were compared by analyses of variance (ANOVA) with permutation tests (Pillar & Orlóci 1996) The test criterion was the sum of square Euclidian distances between groups of sampling units. The same test was used to check for differences in the density of native plants and frequency of zoochorous diaspores between the plantations and the native forest.
We tested whether local assemblages in plantations were a subgroup of species present in native forest with the nestedness metric based on Overlap and Decreasing Fill – NODF (Almeida-Neto et al. 2008). The values range from 0 to 100, where the maximum value represents a perfectly nested assemblage. For this, a matrix of presence/absence of species for each area was generated, and from this matrix the nesting index was calculated. These values were tested by t-test against values created by null models, generated from random resampling of species in sample units, keeping the original richness values of them.
Multivariate analyses of variance (MANOVA) were performed to check for differences in the composition of species and in the trait frequency/mean values among sampling sites. For the MANOVAs, we used the same permutation design as in the previously described ANOVA. Then, principal coordinates analysis (PCoA) was performed in order to graphically characterize the changes in species composition across the areas. The species data were standardized by marginal rows and column totals, and the similarity measure was based on Euclidean distances. A principal components analysis (PCA) was performed to characterize trait variation patterns across the areas based on a correlation similarity matrix. All analyses were performed in MULTIV 2.63 statistical software (available at http://ecoqua.ecologia.ufrgs.br/ecoqua/MULTIV.html).
ResultsSixty-three woody species were counted in the native forest, 40 in the A. angustifolia plantations and 35 in the P. elliottii plantations (species list in Table S2, supplementary material online).
The rarefied richness did not differ between the native forest (14.1 mean species/sample unit ±0.47 standard error) and the two types of plantations (AP=13.4±1.93; PP=11.7±2.53) (Table 1). As expected, the native forest showed a higher density of woody plants (mean 1,041.1 individuals/ha±119.92) when compared to the plantations (355.7 individuals/ha±79.5). The plantations did not differ significantly from each other in relation to the density of woody plants (PP=239.2 individuals/ha±78.2 and AP=472.3 individuals/ha±119.6) (Table 1).
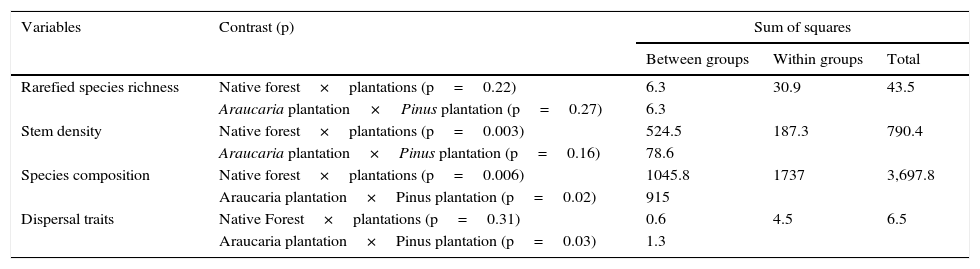
Results of ANOVAs and MANOVAs of rarefied richness, density composition species, and dispersal functional traits measured in a native forest and two different types of plantations (Pinus elliottii and Araucaria angustifolia).
| Variables | Contrast (p) | Sum of squares | ||
|---|---|---|---|---|
| Between groups | Within groups | Total | ||
| Rarefied species richness | Native forest×plantations (p=0.22) | 6.3 | 30.9 | 43.5 |
| Araucaria plantation×Pinus plantation (p=0.27) | 6.3 | |||
| Stem density | Native forest×plantations (p=0.003) | 524.5 | 187.3 | 790.4 |
| Araucaria plantation×Pinus plantation (p=0.16) | 78.6 | |||
| Species composition | Native forest×plantations (p=0.006) | 1045.8 | 1737 | 3,697.8 |
| Araucaria plantation×Pinus plantation (p=0.02) | 915 | |||
| Dispersal traits | Native Forest×plantations (p=0.31) | 0.6 | 4.5 | 6.5 |
| Araucaria plantation×Pinus plantation (p=0.03) | 1.3 | |||
Woody species had not a nested pattern of distribution according to the NODF index (nestedness observed=49.33; nestedness generated=39.40; tcal=1.44; df=6; p=0.19).
The composition of woody species differed between the native forest and the plantations, as well as between the two plantation types (Table 1). Eighteen species of woody plants occurred in plantations but not in native forest, and eight of these occurred only in PP or AP (see Supplementary Material). The first axis of the PCoA scatterplot separated the native-forest and A. angustifolia plantations areas from the P. elliottii plantations (Fig. 1). The second axis showed only 13% of variance and segregated the areas of native forest and P. elliottii from the A. angustifolia areas.
PCoA scatterplot of first and second principal axes of the composition of woody plant species in native forest and Pinus elliottii and Araucaria angustifolia plantations. Sampling areas: ▲=native forest; ■=Pinus elliottii plantations; and ●=Araucaria angustifolia plantations. Species showing coefficient of correlation higher than 0.5 with ordination axis 1 or 2. Algua=Allophylus guaraniticus; Arang=Araucaria angustifolia; Blsal=Blepharocalix salicifolius; Ciamo=Cinnamomum amoenum; Datom=Dasyphyllum tomentosum; Libra=Lithraea brasiliensis; Ludiv=Luehea divaricate; Ocpul=Ocotea pulchella; Parig=Parapiptadenia rigida; Piang=Piptocarpha angustifolia; Qubra=Quilllaja brasiliensis; Scter=Schinus terebinthifolius; Sebra=Sebastiania brasiliensis; Sosan=Solanum sanctaecatharinae; Sytet=Symplocos tetrandra; Syuni=Symplocos uniflora; Vaque=Vasconcella quercifolia.
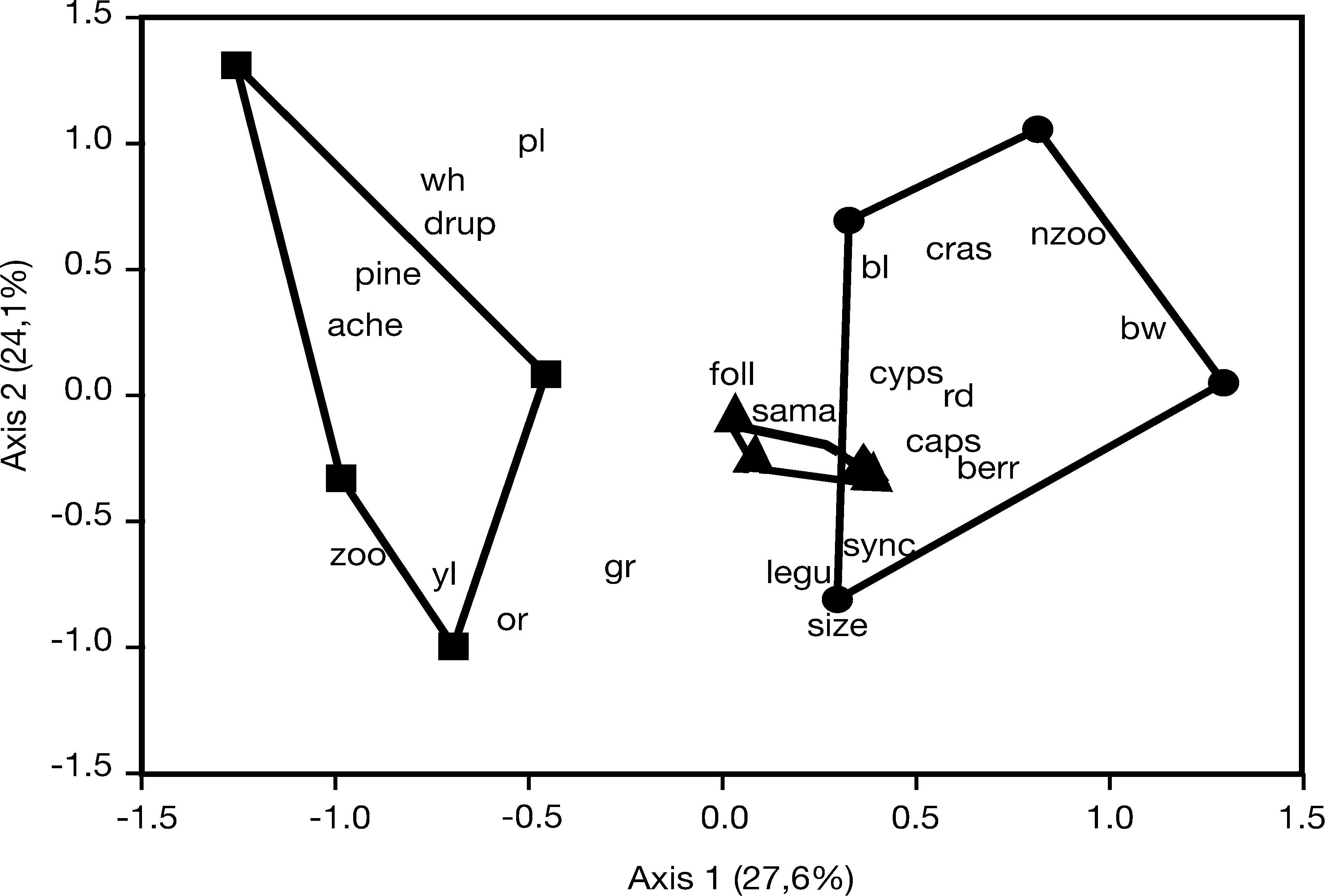
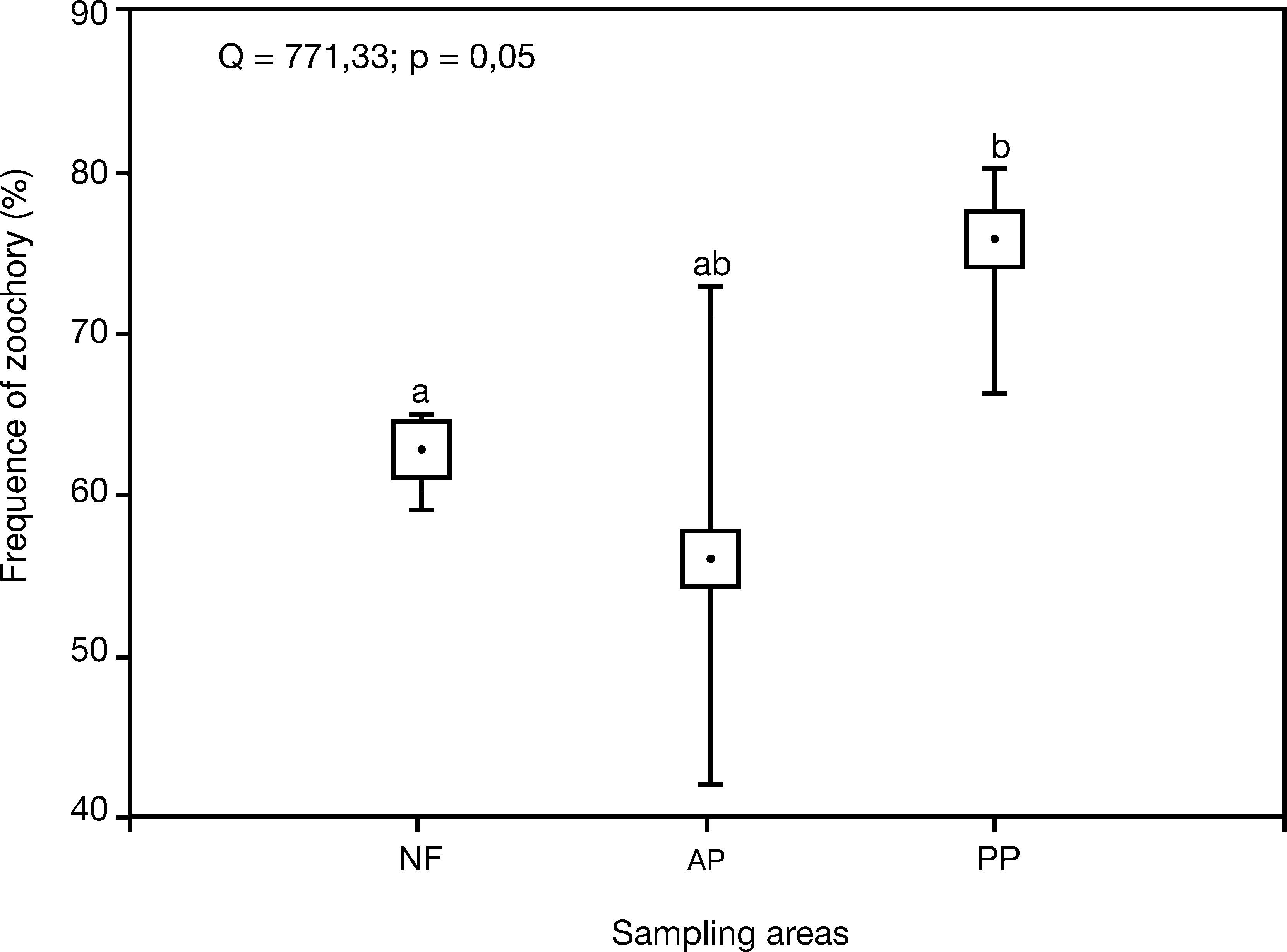
The MANOVA indicated a significant difference in the composition of traits found in the two types of plantations (Table 1). The PCA showed the same pattern as the PCoA (Fig. 2), and again the plantations of A. angustifolia were positioned near the native forest areas. The plantations showed a wider range of dispersal traits compared to the native forest. A higher proportion of zoochorous diaspore traits were found in plantations of P. elliottii when compared to Araucaria plantations and native forests (Fig. 3).
PCA scatterplot of first and second principal axes of the composition of species traits in native forest and Pinus elliottii and Araucaria angustifolia plantations. Sampling areas: ▲=native forest, ■=Pinus elliottii plantations, and ●=Araucaria angustifolia plantations. Traits: zoo=zoochorous fruit; nzoo=non-zoochorous fruit; bw=brown diaspora; rd=red diaspora; yl=yellow diaspora; bl=black diaspora; or=orange diaspora; pl=purple diaspora; gr=green diaspora; wh=white diaspora; size=mean size of diaspore. Types of fruits: legu=legume; drup=drupe; pine=pine; caps=capsule; cyps=cypsela; ber=berry; sama=samara; ache=achene; cras=craspedium; sync=syncarp; foll=follicle.
Frequency of zoochory based on individuals number in the native forest and Pinus elliottii and Araucaria angustifolia plantations. Sampling areas: NF=native forest; PP=Pinus elliottii plantations; AP=Araucaria angustifolia plantations. Different letters indicate significant difference (p<0.05) between the treatments.
Studies across different regions of the world have provided contradictory results regarding woody plant richness within plantations areas (Geldenhuys 1997; Yirdaw 2001; Wang et al. 2012, among others). Some of them have stated that the species cultivated in plantations, which compose the plantation canopy, appeared to affect the abundance of other woody species to a higher degree than species richness (Parrotta 1995; Yirdaw 2001). This might lead to differences in the densities of native woody species found either within plantations of exotic or native trees, leading ultimately to variation in species composition. Indeed, significant differences in understory density between plantation stands with different tree species have been reported (Senbeta et al. 2002). Nonetheless, neither of these studies used rarefaction analysis and thus the differences in richness might be merely an artifact of a difference in the number of individuals. Furthermore, it has been suggested that other factors might influence the occurrence of native woody species within commercial plantations, such as distance from source areas (Martín-Queller et al. 2013), plantation age (Brockerhoff et al. 2003), soil differences due to altitudinal elevation (Wang et al. 2012), and dispersal of colonizer plant propagules (Geldenhuys 1997; Neri et al. 2005). These factors might lead to differences in the density or even in the composition of native woody species occurring in the managed habitats. In the present study, the plantations stands had similar ages and distance from the native forest. Thus, the age of the stands is likely to exert an important effect on the maintenance of similar densities of native woody species in the understories of the two kinds of plantations. Therefore, our first hypothesis, that commercial plantation stands with exotic trees show lower richness and density of native woody species than stands with native-tree plantations and areas of native forests, was partially confirmed, as only plant density differed among the stand types.
Despite the fact that we did not confirm differences in species richness, species composition and trait frequency/mean were different in the planted areas. Furthermore, the composition of woody species found in the understories of plantations did not represent a subset of those found in the native forest, indicating a turnover in species composition. Thus, the second hypothesis was not confirmed in our study. This result is very important for conservation purposes. The plantations might be colonized by pioneer species that did not occur in the native forest, such as Piptocarpha angustifolia (Carvalho 2006) or Schinus terebinthifolius (Backes & Irgang 2004).
Although high frequencies of the zoochorous diaspores in exotic plantations have been found in other studies (Geldenhuys 1997; Onofre et al. 2010; Yirdaw 2001), it was a surprise to discover that the proportion of diaspores with zoochorous syndrome was higher in the P. elliottii plantations when compared to the native forest. Our study confirmed the importance of the fauna for the establishment of understory vegetation in exotic tree plantations, and also suggested the existence of some restriction to the arrival of non-zoochorous diaspores at the understory of P. elliottii plantations. Thus, the species used in commercial plantations may function as environmental filters, in the sense that the stand structure can select for the set of native species that will be able to colonize the plantations (Yirdaw 2001). Possibly the canopy of the P. elliottii plantation exerts a negative influence on the non-zoochorous species, which deserves to be investigated. Therefore, our third hypothesis was corroborated, as woody plants in the native forests showed higher diversity of dispersal traits than Pinus plantations. However, other factors, such as soil conditions, might also determine the higher frequency of zoochorous species within Pinus plantations. Nonetheless, Carmo et al. (2012) demonstrated that the competition with the P. elliottii species did not directly affect the native seedbed regeneration. Our study indicates that Araucaria angustifolia is itself mostly associated with P. elliottii plantations, reinforcing the authors' finding, notwithstanding it did not form the native seedbed.
Data on the regeneration of native species in commercial plantations of A. angustifolia indicated that the animal-based dispersal syndrome showed the highest proportions among plantations of different ages (Barbosa et al. 2009). Seedlings found below A. angustifolia in the highland grasslands of southern Brazil mainly have small black, red, or purple berrylike diaspores dispersed by vertebrates (Duarte et al. 2007). In plantations of this tree, we found higher frequencies of berry, schizocarp, cypsela, syncarp, and capsule types. In general, the type of plantation affects the predominant dispersal syndrome. However, the plantation structure might affect the ability of certain species to complete their life cycles, due to altered habitat conditions. Therefore, it would be necessary to monitor the establishment of colonizer plants in order to evaluate the colonization success. Plantations show a high potential as colonization sites by native woody species, despite their structural differences in relation to native forests. If properly managed, plantations might be catalysts for the recovery of degraded areas (Senbeta et al. 2002; Barbosa et al. 2009), although the structure of plantations may differ from that found in native forests.
AcknowledgmentsWe are grateful for the logistical support of the staff of the Fazenda Florestal Gateados; to Guilherme Seger, Rodrigo Bergamin, Glauco Schussler, Martin Grings, and Letícia Dadalt, for taxonomical plant support; to Andreas Kindel, Luiz dos Anjos, Paulo Antas, Marcos de Souza Lima Figueiredo, and Seth Bigelow, for comments and suggestions. The first author received a doctorate scholarship from CAPES. LDSD and SMH received a fellowship from the Brazilian Research Council – CNPq (grants 303534/2012-5 and 306816/2010-5, respectively).
Supplementary materialSupplementary material associated with this article can be found, in the online version, at www.naturezaeconservacao.com.br.
- Home
- All contents
- Publish your article
- About the journal
- Metrics