Niche germination breadth may determine species occurrence under distinct environmental conditions. We choose Euterpe edulis and Lytocaryum weddellianum to evaluate germination niche breadth under light or dark at water potential of 0MPa, and in either decreasing water potentials (−0.4 and −0.8MPa) or flooded. In contrast to L. weddellianum, E. edulis showed high seed germination at both light conditions and expressive seed germination at low water potentials and flooding. E. edulis had wide seed germination niche breadth and seed addition of the same geographic region might be used for the reintroduction of populations of this threatened palm in different types of habitats of the Atlantic Rainforest. L. weddellianum, however, is unlikely to be able to germinate under altered conditions due to narrower germination niches and might be more vulnerable to extinction if the restricted germination cues no longer occur. Thus, ex situ initiatives might be used for the species conservation.
After seed dispersal, germination is one of the earliest ecological processes that determine the occurrence of a species in a plant community. To recruit a new individual into a population, a set of specific environmental conditions is required for seed germination in the place where seeds are dispersed (Donohue et al., 2010). However, species have different environmental requirements to effect germination (Baskin and Baskin, 2001) and consequently, have distinct germination niche breadths. Species with a broader germination niche breadth germinate seeds in a wider range of environmental conditions than species with a small niche breadth (Luna and Moreno, 2010).
Among distinct environmental factors, light and water are key in determining the location and time of seed germination (Baskin and Baskin, 2001). In moist tropical forests, the light requirement for seed germination is usually more important for pioneer species and small-seeded species (Vazquez-Yañes and Orozco-Segovia, 1993). However, a deficit or excess of water in the soil also affects seed germination (Baskin and Baskin, 2001) and a spatial heterogeneity of water availability occurs within and between tropical forests (Svenning, 2001). Soil water potential has a great heterogeneity within and among tropical forests. In a Bornean lowland rain forest, Ψsoil were higher than −0.05MPa during the wet season; while in the driest periods, the Ψsoil decreased to −0.53MPa (Gibbons and Newbery, 2002). In contrast, the Ψsoil of a dry tropical forest in Mexico ranging from −0.42MPa to −7.31MPa, 60 days after the last rain. However, the evergreen species were restricted to habitats with Ψsoil higher than −1.2MPa during the dry period (Méndez-Alonzo et al., 2013). In general, seed germination of several tropical species is precluded or strongly decreased under low water potentials (Baskin and Baskin, 2001); however, many tolerant species occurring in dry or semi-arid ecosystems germinate seeds under low water potentials (Ψ≤−1.0MPa) (Schütz et al., 2002; Tobe et al., 2006). The ability of seeds to germinate under a low water potential might allow a species to occupy habitats that species with drought-sensitive seeds cannot inhabit (Schütz et al., 2002). In contrast, species that can germinate seeds in flooded conditions might be able to colonize swamp habitats in the forest (Gomes et al., 2006).
Palms (Arecaceae) are abundant plants in tropical moist forests (Henderson, 1995) and their distribution is strongly correlated with light availability and water-related variables (Svenning, 2001; Eiserhardt et al., 2011). Although ecological niche characteristics have a crucial role in determining the pattern of species abundance (Kristiansen et al., 2012), few studies have addressed the interspecific variation in palm germination niche breadth (Orozco-Segovia et al., 2003). Specifically, we are unaware of any studies that have evaluated the seed germination characteristics of palms under low water potentials.
Our aim is to investigate, in palm species, the existence of differences in niche germination breadth in relation to distinct levels of light and water availability under controlled laboratory experiments. We hypothesized that palms with distinct ecological and geographic range have different germination niche breadth. Hence, we choose two species of the Arecaceae (Euterpe edulis Mart. and Lytocaryum weddellianum (H. Wendel.) Tol.) with contrasting ecological characteristics and geographic range, but occurring sympatrically in some areas of the Atlantic Rainforest (Henderson, 2009). Additionally, E. edulis is a vulnerable species due to over-exploitation for the use of the palm heart, named “palmito” (Martinelli and Moraes, 2013).
Materials and methodsThe study was conducted in the ombrophilous Atlantic forest of Guapiaçú Ecological Reserve (REGUA) and Três Picos State Park (PETP) (22°25′02″ S, 42°44′18″ W; Cachoeiras de Macacu, Rio de Janeiro, Brazil). The private reserve of REGUA comprises 7,200ha and overlaps with PETP at 500m of elevation, corresponding to 58,790ha contiguous forest occurring at Serra do Mar. E. edulis is a shade-tolerant species which occur in different vegetation types (e.g. ombrophilous; deciduous; swamp and restinga forests) up to 1000m of elevation along the Atlantic Rainforest biome (Henderson, 2009). L. weddellianum is an understory palm that grows on steep slopes at elevation ranges from 50 to 800m a.s.l., but is restricted to a very small geographical part of the ombrophilous forest of the Atlantic Rainforest biome (Henderson, 2009).
For E. edulis, we collected regurgitated de-pulped fruits from the ground and also ripe fruits from different individuals at the beginning of September 2011 at REGUA. Pizo and Simão (2001) found a similar germination percentage for seeds of E. edulis from de-pulped or ripe fruits. In addition, Leite et al. (2012) demonstrated that seeds regurgitated by small frugivores had a similar rate of seed germination to that of manually defleshed seeds. For L. weddellianum, ripe fruits were collected from at least 15 individuals at the end of October 2011 at Três Picos State Park. In the laboratory, the flesh fruit of both species was removed and seeds were exposed to two light treatments, light and dark at Ψ=0MPa, and also to four treatments with different water availability: Ψ=0MPa, Ψ=−0.4MPa, Ψ=−0.8MPa and flood. We choose these four water treatments because seeds of palms could be frequently subjected to these water potentials in the soils of the distinct ecosystems of the Atlantic Rain Forest Biome. The water potential of −0.4MPa would be probably found during the driest periods in the ombrophilous Atlantic forest; while, Ψ=−0.8MPa might be more predominantly in the deciduous and restinga forests. In addition, it is very common the occurrence of seasonal swamp forests during the wet season in the forests of the Atlantic Rain Forest Biome. Ten replicates each of ten seeds were analyzed per treatment per species. Seeds of E. edulis were sown on filter paper moistened with 5mL deionized water in a 9cm-Petri-dish, whereas the larger seeds of L. weddellianum were sown on filter paper moistened with 10mL deionised water at Ψ=0MPa (light and dark) in a 11cm Gerbox. For the water deficit treatment, seeds were moistened with PEG 6000 solution as in Michael and Kaufmann (1973). For the dark experiments, the Petri-dishes and Gerbox were wrapped in a double layer of aluminum foil. For the flood treatment, seeds were submerged in 40mL deionised water in a Becker for 40 days for E. edulis and 110 d for L. weddellianum. The submersion period in water of L. weddellianum seeds was longer than for E. edulis, since L. weddellianum seeds took longer to onset seed germination in the flood treatment.
Seeds were incubated in a germination chamber at a day/night temperature of 25°C/20°C with a 12h photoperiod. The seeds were inspected every day, except weekends, to count and remove the germinated seeds and to replace the water lost by evaporation. During the dark treatment, we monitored seeds under dim green light (v/ve=0.05μmol of photons m−2s−1). Seeds were scored as germinated when the germinative bottom protruded at least 1mm through the seed coat. When no seeds germinated for 15 consecutive days in a treatment, the experiment was ended. Following the end of the experiment, ungerminated seeds subjected to Ψ=−0.4MPa, Ψ=−0.8MPa and the flood treatment were transferred to deionised water at Ψ=0MPa (light) to test the proportion of seeds that can maintain germinability.
Final germination percentages were calculated and were arcsine square root transformed before analyses. Germination time was calculated as the number of days taken to reach 50% final germination (GT50%). We conducted a two-way ANOVA to assess whether there were significant differences in the final germination percentage between palm species and also among light and water treatments. We excluded the germination time results of Ψ=−0.4 and −0.8MPa and flood treatment of L. weddellianum from the statistical analyses, due to the very low seed germination percentage. Thus, we performed a one-way ANOVA to test the germination time of E. edulis, whereas for L. weddellianum, we performed a t-test for independent samples between the germination time in light and dark treatments. Multiple comparisons were performed using pairwise t-tests, p-adjusted for false discovery rate. All residuals were checked for normality and homoscedasticity. Analyses were implemented in the software package R 2.15.1 (R Development Core Team, 2012).
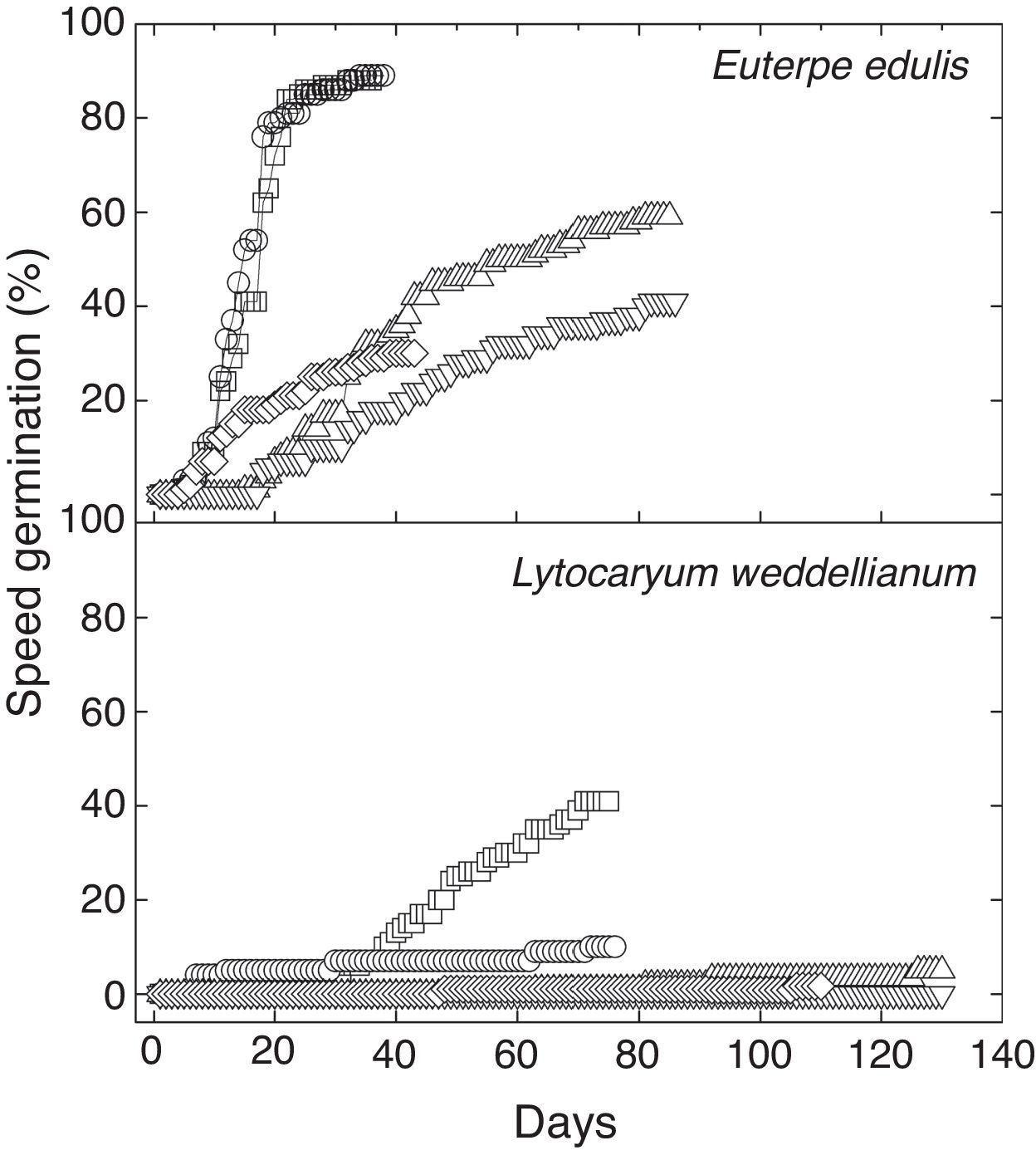
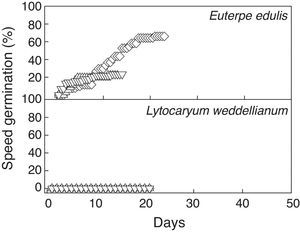
ResultsPercentage seed germination was significantly different between palms and treatments (two-way ANOVA, Fspecies*treatments=5.59, p<0.05, n=10; Fig. 1). Seeds of E. edulis had a high germination percentage at Ψ=0MPa in light and dark. However, mean seed germination was reduced by 31%, 51% and 66% in the Ψ=−0.4MPa, Ψ=−0.8MPa and flood treatments, respectively (Fig. 1; Table 1). For L. weddelianum, higher seed germination percentage was observed at Ψ=0MPa under light conditions (Fig. 1). Nevertheless, mean seed germination of L. weddellianum decreased by 74% in the dark and an extremely low germination percentage was observed in the Ψ=−0.4MPa and flood treatments. No seeds of L. weddelianum germinated at Ψ=−0.8MPa (Fig. 1; Table 1).
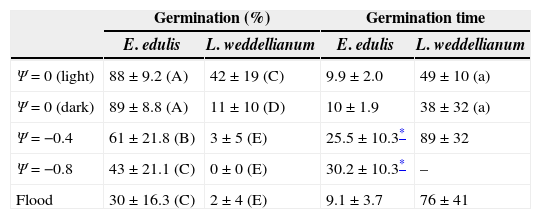
Mean percentage seed germination and germination time (days to reach 50% of final germination ± SD) of Euterpe edulis and Lytocaryum weddellianum in Ψ=0 (light and dark), Ψ=−0.4, Ψ=−0.8MPa and flood treatments. Different uppercase letters represent significant differences in the seed germination porcentage between species and treatments (two-way ANOVA, Fspecies*treatments=5.59, p<0.05, n=10). Different lowercase letters denote significant differences in the germination time of L. weddellianum at Ψ=0 under light and dark (t-test=0.92, p>0.05; n=10).
| Germination (%) | Germination time | |||
|---|---|---|---|---|
| E. edulis | L. weddellianum | E. edulis | L. weddellianum | |
| Ψ=0 (light) | 88±9.2 (A) | 42±19 (C) | 9.9±2.0 | 49±10 (a) |
| Ψ=0 (dark) | 89±8.8 (A) | 11±10 (D) | 10±1.9 | 38±32 (a) |
| Ψ=−0.4 | 61±21.8 (B) | 3±5 (E) | 25.5±10.3* | 89±32 |
| Ψ=−0.8 | 43±21.1 (C) | 0±0 (E) | 30.2±10.3* | – |
| Flood | 30±16.3 (C) | 2±4 (E) | 9.1±3.7 | 76±41 |
Seeds of E. edulis reached the GT50% most rapidly at Ψ=0MPa under light and dark and in the flood treatment (Fig. 1; Table 1). However, seeds of E. edulis subjected to Ψ=−0.4 and Ψ=−0.8MPa showed an increase of 2.5- and threefold in the GT50%, respectively (ANOVA, F=21.79, p<0.05, n=10; Table 1). The GT50% of L. weddellianum was ca. fivefold higher than that of E. edulis at Ψ=0MPa under light (Fig. 1). There was no significant difference in the GT50% of seeds of L. weddellianum between light and dark treatments (t-value=0.92, p>0.05, n=10; Table 1). The GT50% of the seeds of L. weddellianum subjected to the Ψ=−0.4MPa and flood treatments, increased ca. two- and 1.5-fold, respectively. In contrast to L. weddellianum, 23% and 68% of the seeds of E. edulis subjected to 90 days of low water potential and 45 days of flooded conditions remained viable, respectively (Fig. 2).
DiscussionSeeds of E. edulis are insensitive to light and therefore might have higher germination, whether they are placed above the ground or covered by a thick litter layer or buried by animals in the forest soil. Although several seeds of E. edulis are largely consumed by rodents (Pizo et al., 2006), some of them buried for future consumption are frequently forget and might germinate under favorable environmental conditions (Grenha et al., 2010), with greater probability to escape from insect predation (Jansen and Forget, 2001). As observed for E. edulis, most of palms have seed germination indifferent to light (Orozco-Segovia et al., 2003). However, L. weddellianum showed a decrease of 74% in seed germination in the dark compared to the light, probably limiting the germination of buried seeds in the forest. Moreover, seeds of L. weddellianum subjected to Ψ=0MPa under light germinated only 42%. Even in a germination experiment of longer duration (210 days), 59% of the seeds of this palm germinated at Ψ=0MPa under light (G.A. Oda, unpubl. results). In contrast to the tribe of E. edulis (Areceae), the tribe Cocoseae, which L. weddellianum belongs, has been described to have a low seed germination mean rate (37%) (Wagner, 1982).
A large proportion of tropical species have recalcitrant seeds with high water requirement for germination (Vazquez-Yañes and Orozco-Segovia, 1993) and E. edulis is a tropical palm with desiccation-sensitive seeds (Andrade, 2001). However, we still observed expressive seed germination at Ψ=−0.4 and −0.8MPa (61% and 43%, respectively). Additionally, 23% of the seeds of E. edulis that did not germinate under Ψ=−0.4 and −0.8MPa, remained viable. Generally, seed germination at water potentials lower than −0.5MPa has been observed in species that inhabitat semi-arid or arid habitats (Schütz et al., 2002). Few exceptions are found, such as Enterolobium glaziovii, a rare neotropical canopy tree, which still shows seed germination even at very low water potentials of −2.0MPa (Ramos and Andrade, 2010).
It has been suggested that the ability of seeds to germinate at low water potentials might allow the colonization of habitats that drought-sensitive plants cannot inhabit (Braz and de Mattos, 2010). This appears an unexpected finding for palms, which in general are associated with humid habitats (Eiserhardt et al., 2011). However, some species of the Arecaceae, such as E. edulis, occur in microhabitats and in forests with frequent periods of water shortage (Henderson, 1995). In contrast, L. weddellianum has a narrow germination niche in relation to water deficit and no viable seeds of this palm were observed at low water potentials. Thus, seed germination of L. weddellianum might only occur in specific habitats and/or periods of the year with high soil water availability.
The ability of seeds to germinate in flood conditions allows some palms to occur in flooded environments (Svenning, 2001). For instance, Geonoma brevispatha, a palm that inhabitats swamp habitats, showed similar seed germination in both control and flood treatment (Gomes et al., 2006). Seeds of E. edulis showed some tolerance to flooding; 30% of them germinated when submerged in water and 68% of seeds maintained viability after flooding for 40 days, which may favor the occurrence of E. edulis in swamp habitats of the Atlantic Rainforest (Henderson, 1995). As observed for water shortage, L. weddelianum had a narrow germination niche in relation to flooding, probably restricting the occurrence of L. weddelianum to well-drained habitats of the Atlantic forest.
In general, palm seeds require a long time to germinate, because several species disperse seeds with some type of dormancy (Baskin and Baskin, 2001). However, we observed that seeds of E. edulis germinated fivefold faster than L. weddellianum under high water potentials. Rapid seed germination might confer ecological advantages in tropical rain forests, since it minimizes seed exposure to potential predators (Pizo et al., 2006). However, early seedling emergence is only an advantage for seedling establishment when the environmental conditions remain favorable (Braz and de Mattos, 2010). In tropical moist forests, seed germination in many species is delayed in adverse environmental conditions, as was observed for E. edulis, which might increase the probability of seedling emergence during better growth conditions (Vazquez-Yañes and Orozco-Segovia, 1993). Seeds of E. edulis also germinated rapidly in flood conditions, which may favor seed germination at the onset of flooding when still there is a high availability of oxygen in the water column. In contrast to E. edulis, seed germination of L. weddelianum was unexpressive at low water potentials and in flood conditions, which precluded a comparison of the timing of seed germination between species.
The differences in niche germination breadth observed in the two co-occurring palms of the Atlantic Rainforest have important implications for the regeneration of local populations and also for responses to expected future climate changes. Donohue et al. (2010) pointed out that seed of species, such as E. edulis, with a wide germination niche breadth are more likely to germinate under future altered environmental conditions. We should also take into account that in general, canopy and sub-canopy species, as E. edulis, have greater dispersal ability than understory plants such as L. weddelianum (Kristiansen et al., 2012), which might favor recruitment of seedlings of E. edulis in greater spatio-temporal heterogeneity of environmental conditions. Thus, it is a matter of concern that L. weddellianum might be more severely limited in abundance and distribution within the Atlantic Rainforest biome in expected future climate change scenarios because this palm is unlikely to be able to germinate under altered conditions due to narrower germination niches and might be more vulnerable to extinction if the restricted germination cues no longer occur. Thus, L. weddellianum should be the target of conservation initiatives such as conservation ex situ.
In conclusion, greater germination niche breadth of E. edulis under light and water availabilities might allow the use of their seeds to reintroduce a population of this vulnerable palm in a variety of habitats, where the palm has already been extinct due to high palm-heart harvesting pressure. Care should be taken, however, to maintain integrity of the genetic identity of the populations of a certain geographic area. Thus, harvest of seeds must be done in the closest possible populations of the target area of the reintroduction. Although most of palms are often restricted to humid areas in the tropics, seed germination at low water potentials appears to be a promising descriptor of ecological differentiation of species with contrasting ecological amplitudes. Thus, we suggest that variation in germination niche breadth should be incorporated into future studies to improve our understanding of the ecology, management and conservation of palms subjected to spatio-temporal environmental variation.
Conflicts of interestThe authors declare no conflicts of interest.
We are grateful to Nicolas and Raquel Locke and INEA for permission to work in protected areas. We thank Rildo for his invaluable help in the field and Jorge Bizarro for logistical support. Financial support was provided by PAPD fellowship to M.I.G.B; FAPERJ and CNPq to E.A.de.M.