Our study evaluated the anthropogenic threats to the common sloth in the Atlantic forest (AF) by analyzing data collected in wildlife governmental centers. Interesting, new biological data could also be reported. The main risks identified were the road network and falls, followed by domestic dogs and electric shocks. Tourism and preliminary evidences of locally distinct biological features were suggested as potential menaces to conservation of the AF common sloth populations. The birth season within the southeast appears to be the rainy season, but that is not the case in the northeast. Finally, the weight of adult individuals was reported not to vary between sexes. Data were confirmed by the highest number of individuals ever analyzed. Thus, although neotropical wildlife governmental centers usually cope with the lack of human resources, this study highlights that their records can be successfully used to add important biological information to the conservation and study of neotropical wildlife.
© 2014 Associação Brasileira de Ciência Ecológica e Conservação. Published by Elsevier Editora Ltd
The common sloth Bradypus variegatus (hereafter sloth) is highly adapted to forested habitats, rarely leaving the canopy (reviewed by Hayssen 2010). Thus, sloths are extremely forest-dependent, and deforestation is one of the species' major threats (reviewed by Superina et al. 2010).
Despite its slow movements, only a small number of animals hunt sloths, mostly opportunistically (e.g. Vaughan et al. 2007, but also Galetti & Carvalho 2000). However, hunting by domestic dogs, subsistence, medical, artisanal, and illegal traffic activities menace the species across its distribution range (Moreno & Plese 2006; Vaughan et al. 2007; Ballesteros et al. 2009; Noss et al. 2008).
In Brazil, few studies have quantified these additional threats. As far as we could evaluate, only Xavier et al. (2010) highlighted electric shocks in high voltage cables as one of the principal menaces. Evaluating these risks is mandatory to estimate the real degree of threat to sloth populations. This is particularly compelling within the Atlantic forest (AF). Approximately 92% of AF territory has been and continues to be deforested (Myers et al. 2000; SOSMA & INPE 2011). The most populated Brazilian cities are located in this biome. AF protected areas were suggested to be insufficient to preserve its mammalian diversity (Albuquerque et al. 2011). Geographically isolated sloth populations and genetically distinct management units (MUs) are found therein (Phillips et al. 2006; Moraes-Barros et al. 2007). Furthermore, the species’ basic biological requirements are poorly known, especially within AF (Silva et al. 2013a and references therein).
One measure to preserve wildlife resources is the creation of centers for wildlife rehabilitation and management. Generally, these facilities receive, identify, mark, triage, evaluate, recover, and rehabilitate wild animals, either rescued from illegal captures, nature, or captivity (Brazilian normative instruction No. 169/2008; see also Colombian law No. 1333/2009, Peruvian law No. 27308/2000). Within Brazil, there are at least 50 Screening Centers for Wild Animals (CETAS) that comply with these laws (Porto 2009). The Technical Division of Veterinary Medicine and Management of the Wild Fauna (Fauna Division) of the São Paulo Municipality (DEPAVE-SP) also performs a similar work, often in collaboration with other institutions (Moraes-Barros et al. 2006; Porto 2009). Besides rehabilitating and managing animals, these facilities collect individual data from each animal that arrives (e.g. sex, weight, age class, etc.; J.L.S., M.E.L.S., M.B., A.K. pers. comm.). It is our belief that this information has been mostly unexplored.
Thus, our main goal was to use data collected in CETAS along the AF, and DEPAVE-SP, to increase information on threats to sloths and on its biology. Furthermore, this work intended to be a case-study to sustain that active collaboration between neotropical wildlife management facilities, universities, and research centers can contribute to the faunal protection and improve scientific knowledge on species biology.
Material and methodsSeveral CETAS across the AF region and DEPAVE-SP were visited and contacted. For each agency, whenever available, we computed sloth records and classified them according to reason of arrival, date, sex, age class and weight, among others. We also used data from Pernambuco’s CETAS (CETASPE), previously published by Xavier et al. (2010). CETAS-PE data were available in a year-counts dataset of 531 records (2001–2009), and another of 151 monthly-counts (2008–2009; Xavier et al. 2010; Figs. 1 and 2). Descriptive statistics were performed in Microsoft Office Excel 2007 and R 2.15.1 (R Development Core Team 2008). Whenever enough data was available, climatic conditions and tourism records were compared against the sloth records by linear regressions on R. Differences between males’ and females’ weights, and differences between the number of records according with age and sex classes were also tested. Statistical significances were estimated by one-tailed permutation tests; we performed over 1,000 permutations in R
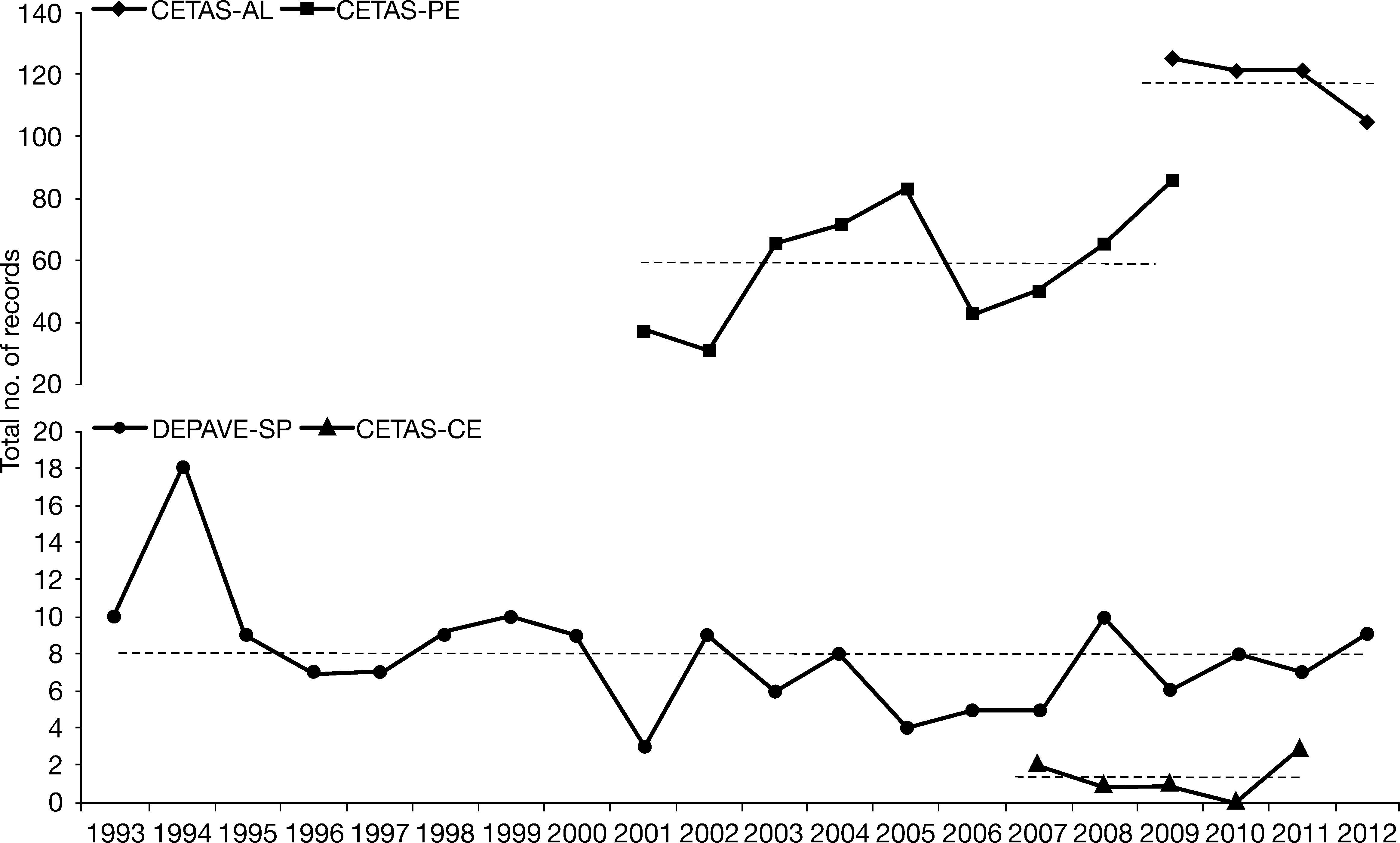
Records for the sloth Bradypus variegatus by year and wildlife facility. Vertical scale is divided in two. Dashed lines correspond to annual means. CETAS – Screening Center for Wild Animals from Alagoas (AL), Pernambuco (PE; Xavier et al. 2010) and Ceara (CE). DEPAVE-SP – Fauna Division from São Paulo (data from November and December of 1992 were excluded).
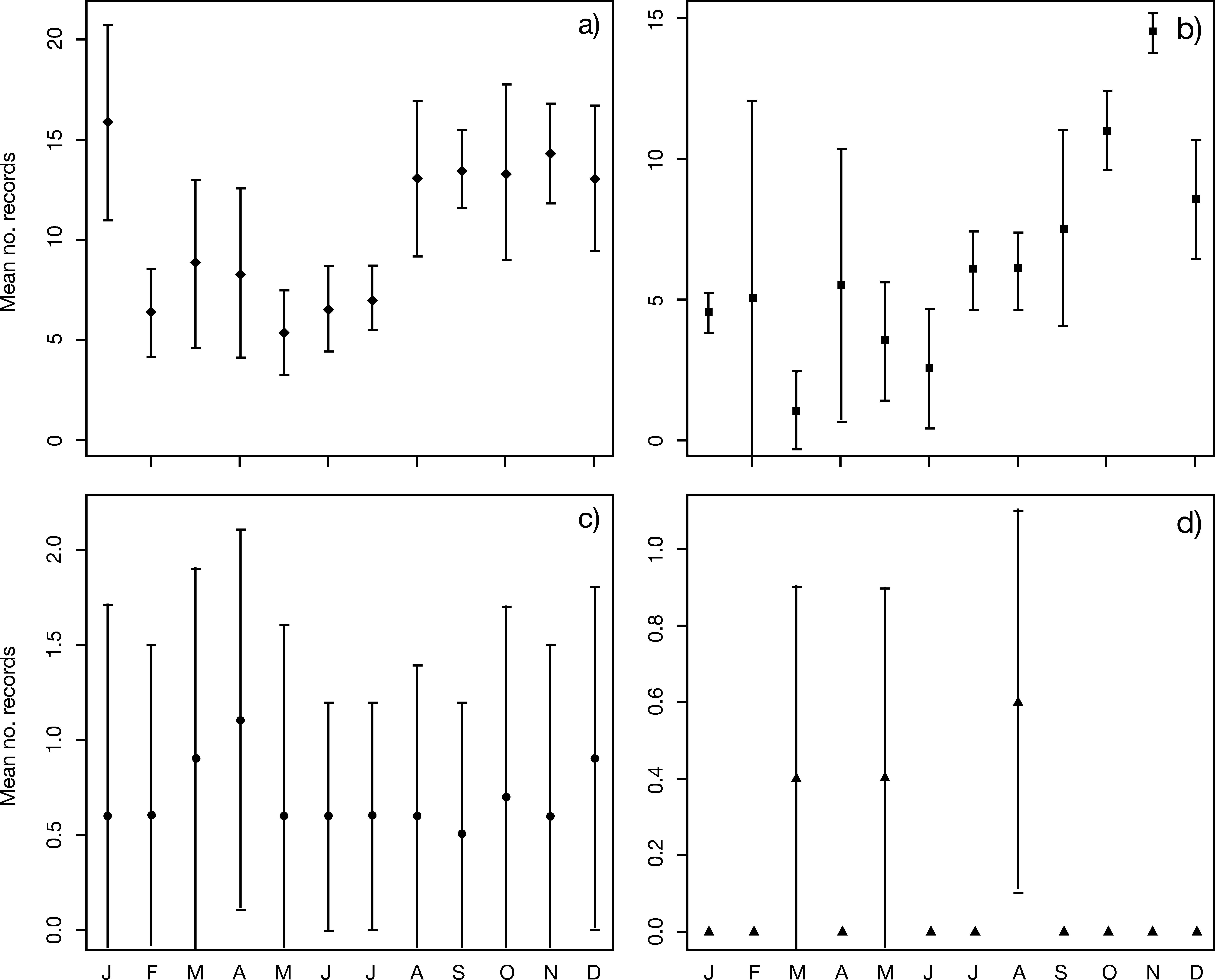
Mean number of records for the sloth Bradypus variegatus by month. Vertical scales are different. a) CETAS-AL (data from 2009 to 2012), b) CETAS-PE (2008-2009; Xavier et al. 2010) c) DEPAVE-SP – Fauna Division from São Paulo (1993-2012), and d) CETAS-CE (2007-2011). CETAS – Screening Center for Wild Animals from Alagoas (CETAS-AL), Pernambuco (CETAS-PE) and Ceará (CETAS-CE).
The Brazilian wildlife governmental centers struggle with two main difficulties: the lack of human resources and the large number of animals arriving, which often need immediate health care and rapid release back in nature. This urgency can hinder the complete data collection (J.L.S., M.E.L.S., M.B., A.K. pers. comm.). An unambiguous cause of arrival was attributed only to 69.94% of the records analyzed. Moreover, some of the animals arriving to the centers can be assigned to a misinformed locality of origin due to unintentional or illegal translocations by locals (J.L.S., M.E.L.S., M.B., A.K. pers. comm.). In addition, a less experienced staff and the poor taxonomic knowledge on some neotropical species (Reeder et al. 2007) can lead to taxonomic misidentifications. Origin and taxonomic misidentifications can bias biological inferences. Despite taxonomic inconsistencies (Moraes-Barros et al. 2011) and illegal traffic affect sloths (Superina et al. 2010), these problems were considered negligible in our data. Doubtful records were eliminated from the analysis. Furthermore, almost all sloths arriving at DEPAVE-SP and some individuals from the three CETAS were genetically characterized under our parallel ongoing projects (Moraes-Barros et al. 2006, 2007; Silva et al. in prep). We found no evidence of recent taxonomic or origin misidentifications affecting these sloths. Microsatellite data analyses allocated individuals to their putative origin with high confidence (Silva et al. in prep). Nonetheless, these evidences emphasize the importance of a direct collaboration between governmental wildlife facilities and different research institutions.
DEPAVE-SP recorded 161 sloths between November of 1992 and December of 2012. Additional 22 routine and sanitary records were registered concerning animals from Parque da Luz, a municipal garden in the city of São Paulo. Approximately 16.4% of the DEPAVE-SP records refer to animals caught more than once; however, this fact was irrelevant for our analyses. No other systematic field expeditions to actively search or capture animals were engaged by this or other facilities. Alagoas’ CETAS registered 472 arrivals (CETAS-AL; 2009–2012) and Ceará’s CETAS registered seven (CETAS-CE; 2007-2011; Figs. 1 and 2).
The average number of sloth records by year were higher in northeastern (CETAS-AL: 118.0±8.9; CETAS-PE: 59.0±19.8) than in southeastern and northern facilities (DEPAVE-SP: 8.0±3.1, CETAS-CE: 1.4±1.1, respectively; Fig. 1). Probably, these differences resulted from a combination of several factors. We observed a greater awareness to the occurrence of sloths and a higher contact between locals and these animals in northeast in comparison with the southeast. Silva et al. (2013a) estimated higher sloths’ density in a northeastern AF botanical garden than in a southeastern natural park. The relative amount of area under the jurisdiction of the agencies and its degree of protection must also be considered. The state of Alagoas (AL) has approximately 150.000ha of AF remnants, representing 5% of its territory (SOSMA & INPE 2011). However, only 0.18% of the AL territory is a protected area. This is the least protected state represented in our data (IBGE 2011), and the one that receives the highest number of sloths (Fig. 1). The state of Pernambuco (PE) has only over 80,000ha of AF remnants than AL (SOSMA & INPE 2011). Nevertheless, this corresponds to a bigger percentage of protected area (around 4.4%; IBGE 2011). On average, CETAS-PE has received only a third of CETAS-AL individuals. DEPAVE-SP received the lowest number of sloths (eight per year), on average. However, the state of São Paulo (SP) has the biggest AF remnants area (over 2.6 million ha), corresponding to 11% of its territory, with the highest absolute and relative amount of protected area (SOSMA & INPE 2011; IBGE 2011). The state of Ceará (CE) is a different case, since its predominant natural habitat is Caatinga, a savannah-like biome. AF remnants represent only around 1% of its territory (SOSMA & INPE 2011), which appears to be the main reason for the limited number of sloths arriving CETAS-CE.
Fig. 2 presents the mean number of arrivals recorded by each facility. Month-by-month analysis demonstrated that CETAS-PE and CETAS-AL received more animals during the dry season (from August/September to December/January; Fig. 2a-b). A different trend appears to be predominant in the southeast. DEPAVE-SP receives more individuals during December, March, and April (Fig. 1a), corresponding to the beginning and ending of the rainy season in southeastern Brazil (INMET 2011). The small amount of records in CETAS-CE limited similar inferences (Fig. 2d).
In CETAS-AL and CETAS-PE, both annual and monthly increases in the number of records appear to correspond to increases in local tourism (EMBRATUR 2013; reports from 2005-2013), but no statistically significant correlation was found (p>0.05; data not shown). Keeping animals in captive and selling photographs of tourists holding it is common practice in northeastern Brazil (Superina et al. 2010). Thus, further investigation on the influence of tourism on northeastern AF sloths population is needed.
Differences in the number of records between sexes were not statistically significant for DEPAVE-SP and CETAS-AL datasets (p=0.692 and p=0.772, respectively; Fig. 3). This is in agreement with studies reporting sloths’ sex ratio close to 1:1 (Hayssen 2010), so may reflect natural population demography. Regarding age classes, adults were more frequent than juveniles and offspring in both DEPAVE-SP and CETAS-AL (Fig. 3; p<0.05).
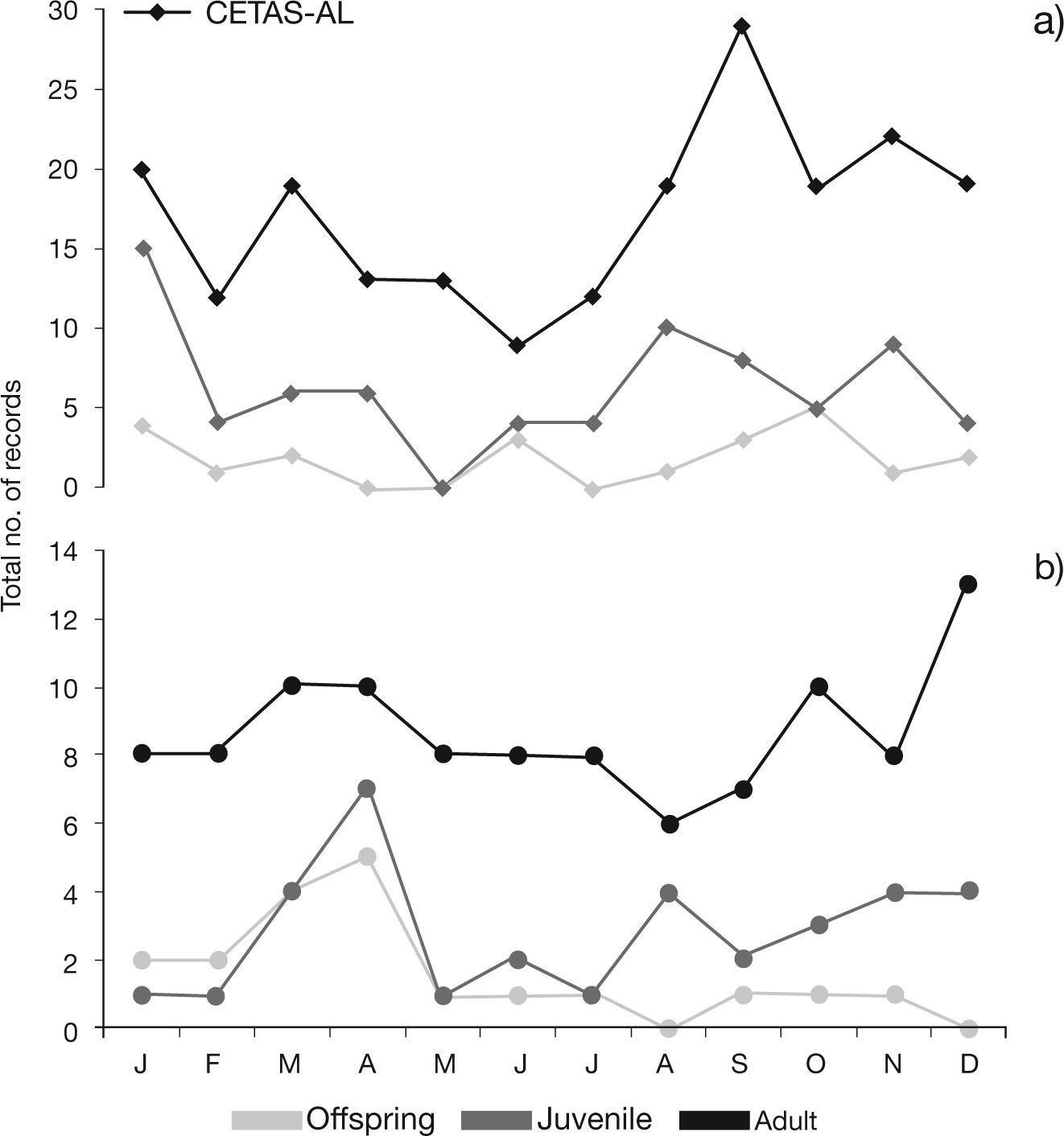
Records for the sloth Bradypus variegatus, by age and sex classes in a) CETAS-AL – Screening Center for Wild Animals from Alagoas (2009-2012) and b) DEPAVE-SP – Fauna Division from São Paulo (November 1992-2012). Vertical scales are different. (F=females; M=males; A=adults; J=juveniles; O=offspring).
Previous data on sloths’ reproduction indicates births’ seasonality, limited to the rainy season (Hayssen 2010). Noteworthy, offspring arrived to CETAS-AL and DEPAVE-SP during almost all year (Fig. 4). During Parque da Luz management activities, DEPAVE-SP registered two births in December of 2006 and February of 2008, and a pregnant female was caught in August of 1994, supporting the rainy season as the birth season within southeastern AF. Also, the number of offspring arriving at DEPAVE-SP peaked at the end of this season (Fig. 4b). However, Bezerra et al. (2008) contradicted this seasonality by reporting a sloth copulation event during the rainy season in northeastern AF. Data collected by CETASAL appear to also disagree, since several peaks of offspring’ arrivals were recorded (Fig. 4a). These contrasting observations suggest that sloths’ biology in the AF is still unclear, and more studies are needed. It is particularly important to investigate potential biological differences in AF sloth populations, since genetically differentiated MUs were identified in the biome (Moraes-Barros et al. 2007; Silva et al. in prep)
Approximately half of the offspring registered was already dead or eventually died in the facilities (43.90%; 18 of 41). Food requirements are difficult to be addressed, because individuals are quite specialists, and the behavior iss passed from the mother to the offspring (Hayssen 2010). Thus, it is difficult to maintain these animals properly fed while captive. Nevertheless, CETAS-AL has made progresses, maintaining an offspring for more than four months in captivity (Silva et al. 2013b).
Overall, only 14.69% (68 of 463) of the individuals’ outcomes refers to dead animals. From those reported dead, the death of only three individuals was attributed to road kill (about 6% of all obits), but roads were the most important menace. Thirty-seven records in DEPAVE-SP and 36 in CETAS-AL corresponded majorly to animals found crossing or close to streets, highways, and railway tracks. Falls were highlighted by Ballesteros et al. (2009) as the main reason for animal mortality in the Viento Solar Natural Reserve, in Colombia. The authors did not quantify this evidence, but, in our study, 31% of the animals with history of fall died or arrived dead at the DEPAVE-SP. This was the second main reason for animals’ arrival in this facility (45 records), and 27% of the times it was the registered cause of death . Even though all individuals with visible injuries from electric shocks were already dead when collected (four records on DEPAVE-SP), these accidents represented only 7.8% of the registered cause of death. Xavier et al. (2010) emphasized the importance of this type of accidents, but did not present data for other menaces. Finally, only three individuals were reported to have been attacked by dogs in DEPAVE-SP, but none lethally.
DEPAVE-SP recorded weight for 94 adult animals (59 males, 33 females). Males weighted 4.90kg (±0.819) and females 4.66kg (±0.906). This difference was not statistically significant (p=0.904). Additionally, during a field expedition in 2004, NMB and MELS weighted all sloths found in a square in the city of Teófilo Otoni, Minas Gerais (MG), central AF. Again, the weight of adult males (3.96kg±0.793; n=10) was similar to that of adult females (4.18kg±0.559; n=9; p=0.752). The number of animals measured (the highest number ever analyzed in a study) enabled us to discard the weight as a sloth sexual dimorphism characteristic, found for other Bradypus species (Lara-Ruiz & Chiarello 2005). Adult sloths in central AF weight on average 4.1kg (2.5 to 5.0kg), and in southern AF, 4.8kg (2.5 to 6.5kg). Due to the different amount of animals weighted in both regions (19 and 94, respectively), these results are presented separately, and no statistical test was performed to compare them. Similar measures were previously reported for Nicaraguan and Panamanian specimens (Hayssen 2010).
Decades of records on sloths collected by wildlife governmental facilities add evidence on the birth period of the sloths within AF, and new data regarding the weight of individuals within the biome could be collected. Most interestingly, this study identified important topics that can jeopardize future conservation actions of these populations if not accounted for, such as the influence of local tourism and the possibility of distinct local behavior. We validated the data collected on wildlife governmental centers as an important source of wildlife information. Nevertheless, it does not replace data from studies in the wild. We confirmed that empowering the wildlife governmental centers with sufficient human resources, namely with the establishment of long-term collaborations with universities, and synchronizing activities, knowledge, and experiences among centers will improve work, encourage scientific production, facilitate conservation, and increase biological knowledge on neotropical wildlife.
The authors would like to thank to Camila L. Clozato, all the CETAS and DEPAVE-SP staff, and their collaborators, namely the Fire and Police Departments, health and environmental institutions, and citizens, who have been collecting animals and data across the years. We also acknowledge two anonymous reviewers for their helpful comments on an early version of this manuscript. S.M. Silva was supported by a Foundation for Science and Technology PhD grant (SFRH/ BD/40638/2007). N. Moraes-Barros was supported by CAPES. J. S. Morgante had a research grant from FAPESP (08/52207-0).