Deer species play a key role in processes that maintain ecosystem community structure and composition. However, several of the species are considered at risk of extinction. One of the strategies for their conservation is the maintenance of legally protected areas in which administration is prescribed by a management plan. Species inventories form the basis of this instrument as it allows to identify key resources and taxons. We reviewed the management plans of Brazilian protected areas to assess the suitability of methods employed to identify deer species. We reviewed 118 management plans (covering 298,000 km2 of protected area) and checked each occurrence record for information regarding detection methods, which we classified according to their suitability for species-level identification. We found that 38% of the records did not report the species detection method used. Specific identifications were obtained through seven different methodologies, of which 12% were considered suitable, 28% were suitable with caveats, and 60% were unsuitable for species identification. We conclude it is necessary to improve the scientific rigor applied to inventories and data collection to avoid producing further inconsistent information, as this might affect management actions and risks the exclusion of threatened species from public policies on conservation planning.
Neotropical ungulates play essential ecological roles in their ecosystems, including seed dispersal, herbivory, soil movement, and providing food resources for different predators species (Taber et al., 2016; Beck et al., 2013; Peres, 2000). Despite their biomass and innumerable interspecific interactions, the group faces a lack of ecological data as demonstrated by scarcity of studies conducted for forest ungulate species since the 2000s (Taber et al., 2016). The knowledge deficit regarding their distribution and community composition compromises management policies, potentially leading to ineffective conservation actions and, consequently, to a greater chance of the species’ extinction and environmental disturbance.
Deer are the most diverse group of Neotropical ungulates and neotropics represents a diversity hotspot for the Cervidae family. Seventeen species occupy a large set of different habitats, such as tropical forests, savannas, mountain landscapes, and grasslands (González and Duarte, 2020). According to the latest International Union for Conservation of Nature (IUCN) Red List, 59% (10/17) of Neotropical deer species are classified in threatened categories, which represents a worse scenario compared to mammals in general (Schipper et al., 2008; IUCN, 2021). IUCN Red List has been extensively used to guide conservation planning and business decision-making (Rodrigues et al., 2006; Bennun et al., 2018) through a consistent evaluation process to identify species conservation status, threats and priorities (Mace et al., 2008) Brazil harbors not only the major part of deer diversity in the continent, but most of the threatened species too: three of the eight deer species that occur in Brazil — Blastocerus dichotomus, Mazama nana, and M. bororo — are considered to be vulnerable (IUCN, 2021). Habitat loss, diseases transmitted by domestic animals, the presence of dogs in native areas, and hunting have represented the main threats to these species (González and Duarte, 2020).
One of the main strategies for long-term biodiversity conservation focus is the establishment of protected areas (Aichi Target 11; Convention on Biological Diversity, 2006). Protected areas (PAs) contribute significantly to species conservation and their effectiveness is directly linked to management activities and funding (Bruner, 2001). PAs are geographic spaces, designated and managed through legal measures, whose purpose is to conserve nature over the long term (Thomas and Middleton, 2003). Brazil has one of the greatest PAs national systems in the world (Chiaravalloti et al., 2015) and they are administered (and thus managed) by federal, state, or municipal governments, as well as private landowners (Law 9.985; Brazil, 2000).
The governance and all technical aspects of a protected area administration (surveillance, public use, research, conservation management, etc.) are prescribed by a Management Plan (MP). This document presents regional and local analyses to identify key features and to establish the management objectives and actions to be implemented in a PA (Thomas and Middleton, 2003). Regarding biodiversity information and conservation actions, MPs are expected to include species occurrence through literature information or primary data from fauna and flora species inventories (Thomas and Middleton, 2003). The correct species diagnosis is crucial for the identification of threatened or human-exploited species that deserve special attention and specific management actions. Unfortunately, half of Brazilian PAs are not sampled to record species occurrence, only 1% is considered well sampled (Oliveira et al., 2017) and independent scientific research along Brazil’s PAs is rare and biased (Gonçalves et al., 2021). In this context, primary filed data used in the MP inventories are of great importance to guide conservation planning in this megadiverse country.
Inventories containing primary data records are based on species detections and identifications. They are essential to assist in decision-making on population management and conservation (Lewinsohn and Prado, 2005; Mace et al., 2008). However, taxonomic determination can be a challenge given the difficulties associated with some methodologies and the experience necessary for the correct identification of species (Austen et al., 2016; Shea et al., 2011). Additionally, morphological similarity, evasive behaviour, and the rarity of some taxonomic groups can compromise not only detection but also the identification of certain species. However, the frequency of, and circumstances behind, erroneous species identification are not well established (Elphick, 2008). Consequently, important questions surround the accuracy of species records, and possible errors must be considered when information is used to support decision-making (Austen et al., 2016).
Therefore, the use of inappropriate fauna survey methods may originate unsound community composition lists and skew information regarding the conservation status, distribution, and temporal trends of species. Thus, it probably compromises the effectiveness of public policies regarding species management and conservation in protected areas. Neotropical deer are an interesting group among medium and large mammals to address this issue, given their ecological and morphological diversity, different degrees of detectability, and the presence of cryptic species (Cifuentes-Rincón et al., 2020; González and Duarte, 2020). Besides, recent studies demonstrated the limitations of using indirect records such as faeces and footprint morphology to distinguish deer species (Angeli et al., 2014; Costa et al., 2017). In this context, our objectives were to examine the methods used in Cervidae detection in management plans to access the reliability of species identification for this taxon in protected areas of Brazil, as it should reflect the Neotropical region situation.
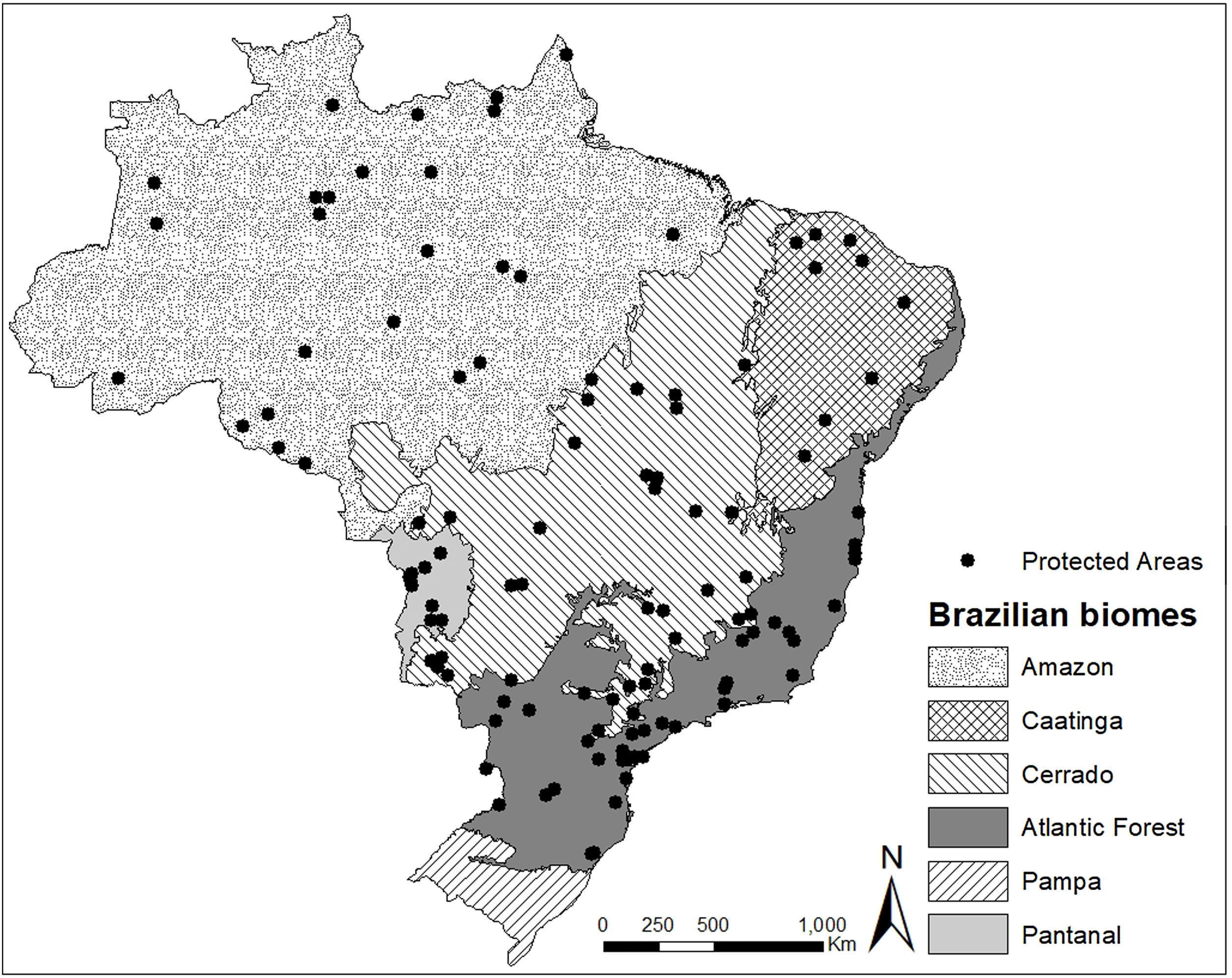
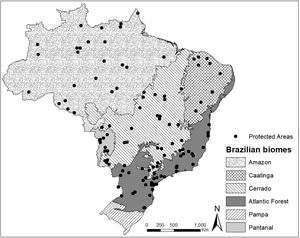
Material and methodsData acquisitionWe selected the management plans available in 2019 of the 25 largest protected areas under public authority and the 25 largest protected areas under private authority (of at least 100 ha) in five Brazilian biomes: Amazon, Cerrado, Caatinga, Pantanal, and Atlantic Forest. For the biomes with fewer than 25 protected areas, or with protected areas that lacked management plans, we used the largest possible number of areas. Following these criteria, we evaluated 118 management plans containing deer records, representing a total protected area of 298,000 km2 (≈20% of all the protected area in Brazil). For the Amazon biome, we evaluated 25 management plans (all protected areas under public authority); for the Atlantic Forest, 39 management plans (25 public protected areas and 14 private protected areas); for the Pantanal, 10 management plans (2 public protected areas and 8 private protected areas); for the Caatinga biome, 9 management plans (6 public protected areas and 3 private protected areas); and for the Cerrado biome, 35 management plans (25 public protected areas and 10 private protected areas) (Fig. 1).
When analysing the selected management plan (complete list in Supplementary Material-C), we summarized the records of Cervidae species occurrence and the species detection method used. When the management plan did not report the detection method directly, we searched for its description in the text and in the cited literature, when available. If the method could not be found, we classified the method as unreported. Some occurrence records relied on more than one detection record, using different methods. Summarizing the occurrence data and the respective detection methods allowed their usage frequencies to be analysed.
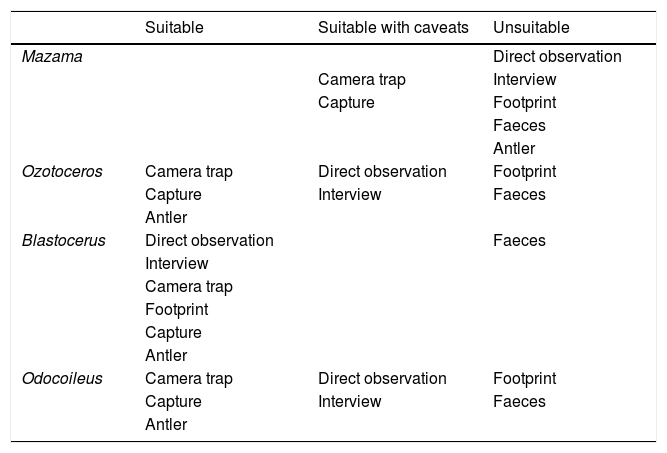
Record suitabilitySeven detection methods for identifying deer species were cited in the management plans: capture, camera trap, direct observation, interview, antler, footprint, and faeces. We categorized each detection method per genera in three suitability categories: “suitable”, “suitable with caveats”, or “unsuitable” (Table 1). This classification was based on the existing literature and the authors' long experience, as described below. First, it is important to state that we did not base this classification of suitability on the geographical distribution of species or the possibility of species co-occurrence. Identification of records must be reliable per se and should be the primary information source for assessing occurrence and geographic distribution.
Classification of detection methods according to their suitability for each genus of ungulates that occur in Brazil.
| Suitable | Suitable with caveats | Unsuitable | |
|---|---|---|---|
| Mazama | Direct observation | ||
| Camera trap | Interview | ||
| Capture | Footprint | ||
| Faeces | |||
| Antler | |||
| Ozotoceros | Camera trap | Direct observation | Footprint |
| Capture | Interview | Faeces | |
| Antler | |||
| Blastocerus | Direct observation | Faeces | |
| Interview | |||
| Camera trap | |||
| Footprint | |||
| Capture | |||
| Antler | |||
| Odocoileus | Camera trap | Direct observation | Footprint |
| Capture | Interview | Faeces | |
| Antler |
Indirect records were classified based on empirical tests of morphological descriptions and morphometric analysis using faeces (Costa et al., 2017) and footprints (Angeli et al., 2014). Those studies showed high overlap and low discriminating power among Brazilian deer species with few exceptions. Thus, the use of this kind of indirect record was considered unsuitable for species identification in the studied region (Angeli et al., 2014; Costa et al., 2017).
Antler morphology in Neotropical deer is well described and should therefore function as an effective indicator, despite the fact antlers are male-exclusive in the analysed species. Their basic structure was recently summarized by Heckeberg (2020) in an extensive analysis of Cervidae morphology. The pampas deer (O. bezoarticus) has a three-tined medium-sized antler whilst white-tailed deer (O. virginianus) and marsh deer (B. dichotomus) have more complex antlers with multiple tines that increase in number in each renovation cycle (Heckeberg, 2020). Nevertheless, the antlers of these species are quite distinct. White-tailed deer are much smaller in size in the tropic region than those in North America, which is reflected in its antlers size (Gallina et al., 2010). In its turn, marsh deer has larger antlers, both in size and number of tines, which are never pointed inward as in Odocoileus, that even attain more than ten points in an adult male (Piovezan et al., 2010). Finally, brocket deer of the Mazama genus have spike-like, single-tined antlers, with all species displaying a similar size and structure (Rossi, 2000), which makes it challenging to differentiate the species by this character alone.
Methods that rely on morphological discrimination (capture, camera trap, direct observation, interview) are at risk of inaccuracy because size, colour and pelage can be confused between some species. Therefore, these methods are especially affected when based on sub-optimal records such as imperfect photographs and sightings that prevent a detailed inspection. Marsh deer should represent an exception in this context because of their long reddish pelage, long black legs, and large size compared to other species (Piovezan et al., 2010). The method characterized as “capture”, which is understood as an animal’s body being “in hand” (as an actual capture, road-kill, or a found carcass) should produce a more correct identification, as it is possible to observe details and take measurements of the body or bones. Morphological analysis can distinguish marsh deer from pampas deer, and both from brocket deer, by observing, for example, the orbital region (Merino et al., 2005). Morphological identification becomes particularly problematic among Mazama species (comparative images in Supplementary Material B), even with “captured” animals, as demonstrated in museum specimens (Mantellatto et al., 2020). Using genetic analysis these authors found a high frequency of misidentifications in vouchers from the Atlantic Forest. In addition, morphometric analysis focusing on taxonomy and ecomorphology found great overlap between Mazama species (Cassini and Toledo, 2021; Cifuentes-Rincón et al., 2020; Duarte et al., 2008; Merino et al., 2005). Finally, Vogliotti (2003) reported that the same morphological problems, among other issues, render interviews equally susceptible to error. For example, respondents identified antlerless female deer as a separate species from males (Vogliotti, 2003).
ResultsOur review of 118 management plans found 232 occurrence records of deer and in 38% of them (n = 88) the detection method was not reported. The species frequency in the occurrence records was as follows: M. americana (n = 84), M. gouazoubira (n = 77), O. bezoarticus (n = 29), B. dichotomus (n = 20), M. nana (n = 11), M. bororo (n = 5), M. nemorivaga (n = 4), and O. virginianus (n = 2).
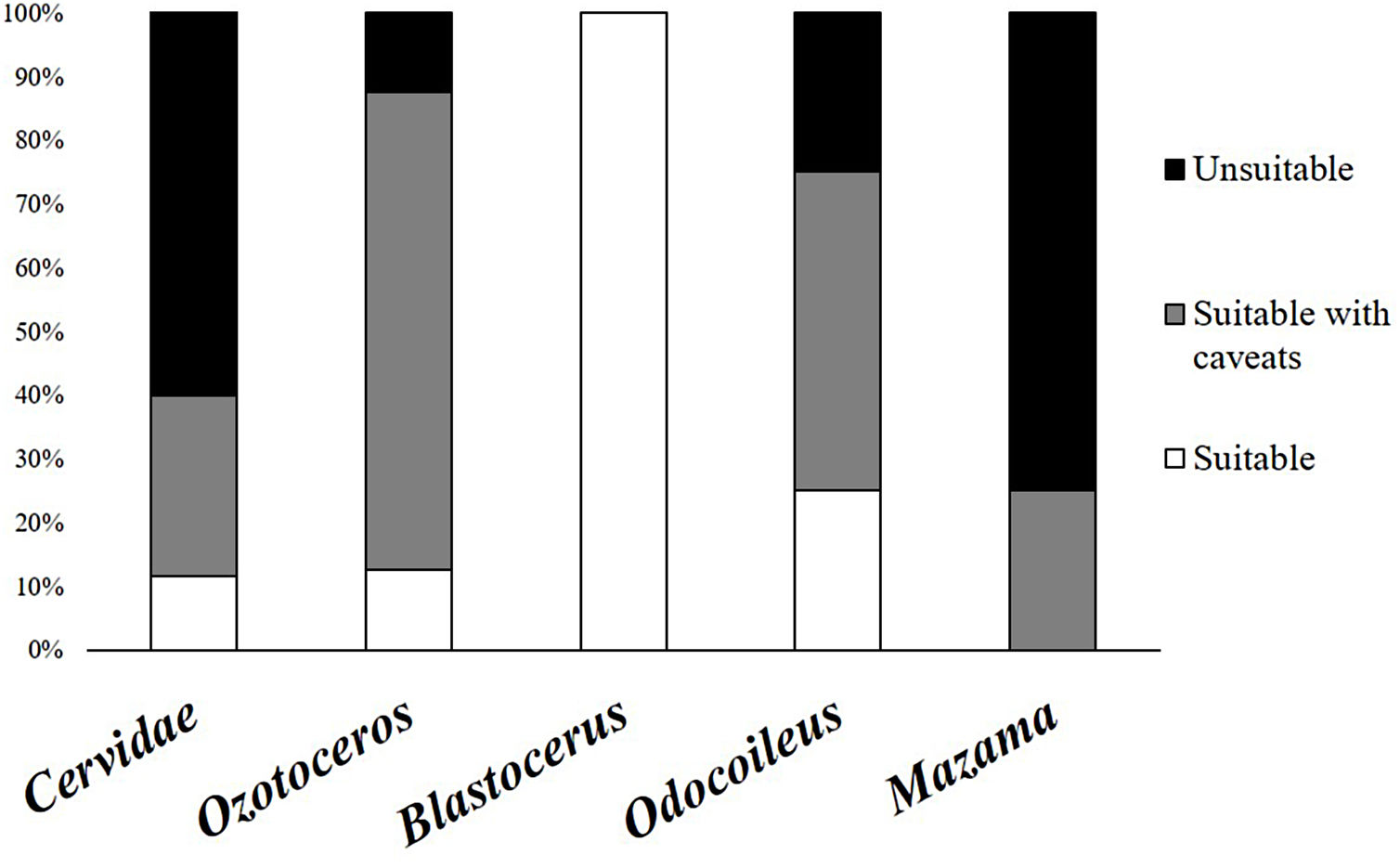
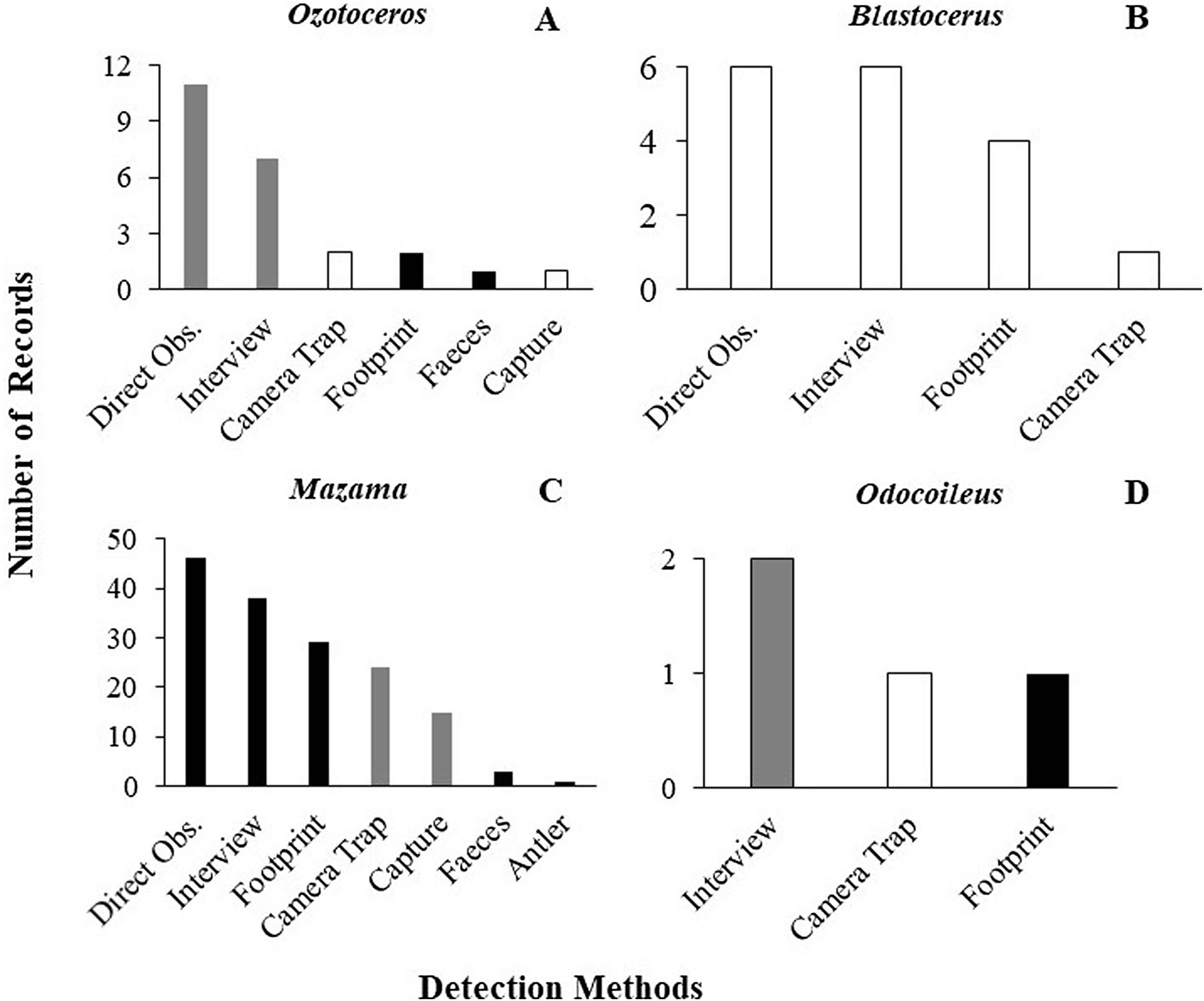
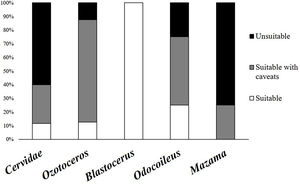
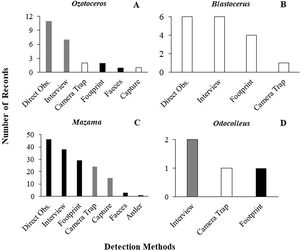
As previously mentioned, an occurrence record of one species in a protected area may be based on more than one detection method. The 144 occurrence records that reported their methods were based on 201 detection records. When these detections records were analysed, 60% were found to have used methods considered unsuitable, and 28% used methods that carry caveats regarding their application. Only 12% used suitable species identification records and could be considered trustworthy. The same analysis found large differences between the suitability of identification in Cervidae genera (Fig. 2). The methods most frequently used for detection of deer species were direct observation (n = 63), interviews (n = 53), and footprints (n = 36) (Fig. 3).
Percentages of detection records, shown for all Cervidae family and groups, in each suitability category (suitable methods in white, suitable methods with caveats in gray and unsuitable methods in black) according to the used method to detect and identify deer species in protected areas of Brazil.
Frequency and suitability of methods (suitable methods in white, suitable methods with caveats in gray and unsuitable methods in black) used for detection and identification to species level in the different genera of Cervidae in protected areas (A) Ozotoceros; (B) Blastocerus; (C) Mazama; (D) Odocoileus.
Analysing the occurrence records and their coherence with the geographic distributions described by the IUCN Red List (IUCN, 2021), we found incongruencies in the Caatinga biome (complete data and geographic distributions in Supplementary Material A and D). Both M. americana and O. bezoarticus were reported as detected in protected areas located in the Caatinga, outside their geographic distribution. We also found records of M. gouzoubira in the Amazon biome, a region in which the species does not occur. This case, however, is derived from the taxonomic revalidation of M. nemorivaga for the Amazon (Rossi, 2000).
DiscussionThe suitability classification identified in the present study should be considered a conservative approach to correctly identifying deer species. Among the most commonly used methods, direct observation and interview play a major role because of their low costs and ease of use. Identification of species by sight can be problematic because the ability to detect and correctly identify an animal depends on the experience of the observer, which is difficult to evaluate (Austen et al., 2016; Fitzpatrick et al., 2009; McClintock et al., 2010). Further, indirect records are widely used because the data are easy to collect, but morphometric identification of footprints and faeces has been questioned for deer (Angeli et al., 2014; Costa et al., 2017). Compared to other taxa, indirect records of deer presence are based on very simple structures (hooves and pellet faeces), which do not provide sufficiently powerful quantitative characteristics for accurate discrimination. In the case of sympatric species of Mazama, discrimination is critical (Angeli et al., 2014; Costa et al., 2017). To exemplify how problematic indirect records can be, faeces from the marsh deer (∼100 kg) showed a significant measurement overlap with faeces from other smaller species (e.g. M. americana, M. bororo, and O. bezoarticus ∼25–40 kg; Rossi, 2000) despite discrepancies in body size (Costa et al., 2017).
Our results evidenced the particular difficulty of identifying Mazama species, a key ungulate group that merits special consideration because of their elusive behavior that prevents it from being captured (Vogliotti and Duarte, 2009). In addition, the morphological similarity among brocket deer species, derived from their evolutionary convergence while adapting to forest environments, presents a major challenge for studying the group (Cassini and Toledo, 2021; Duarte et al., 2008). Indeed, some species that have already been described as clearly genetically differentiated cannot be identified taxonomically by their morphological characters and cryptic complexes have been characterized (Cassini and Toledo, 2021; Cifuentes-Rincón et al., 2020; Duarte et al., 2008; Merino et al., 2005). Fourteen per cent of the management plans opted, appropriately, to identify only the genus (Mazama spp.) when reporting the presence of Mazama — the most technically correct position given the lack of suitable methodologies for identifying the species. The most appropriate approach to correctly identify a Mazama species presence is genetic identification of biological samples and this should be encouraged once the M. americana and M. nemorivaga cryptic complexes will probably be reviewed and split into different species shortly (Cifuentes-Rincón et al., 2020; González and Duarte, 2020). Brocket deer accurate identification is a major issue because six of ten species are threatened, like the two endemic species of Atlantic Forest M. nana and M. bororo. Management actions effectiveness might be compromised if the erroneous identification of a threatened species ignores its presence (false negative) or expand its occurrence area (false positive).
Identification errors are common in species inventories, and the effects of such errors have already been evaluated in surveys of mammals, birds, anurans, fish, plants, and insects (Austen et al., 2016; Garcia-Vazquez et al., 2012; McClintock et al., 2010; Scott and Hallam, 2003). The accumulation of false positive errors can skew population estimates, distribution data, and occupancy modelling (Beerkircher et al., 2009; Miller et al., 2011; Molinari-Jobin et al., 2012). The most serious consequence of this kind of bias is a distorted assessment of the extinction risk that a species faces since geographic distribution and population connectivity are considered for the IUCN Red List criteria (Mace et al., 2008). IUCN Red List uses distribution data (extent of occurrence and area of occupancy) directly as the main criterion (Criterion B: small range area and decline) and as subcriteria (Criterion C2 and D2) to estimate extinction risk (IUCN, 2012). A broader distribution with mature individuals equally distributed among different populations would result in a lower extinction risk than minor distributions with individuals concentrated in one or few populations. For example, misidentification led to an underestimated threat level for the white marlin (Tetrapturus albidus), causing mismanagement of the fish stock, which may have contributed to its overexploitation and population bottleneck (Beerkircher et al., 2009). Likewise, distribution data contaminated by misidentifications of the lynx (Lynx lynx) were detrimental to understanding the level of risk to this species (Molinari-Jobin et al., 2012).
False-negative occurrence (missing a threatened species) by its turns, compromises management actions that could protect target species. A protected area with the confirmed presence of a threatened deer should give priority, for example, to the health of any herds of domestic ungulates in surrounding areas and to control the presence of feral dogs (González and Duarte, 2020). Cervids are particularly susceptible to infections that are generally unapparent or very mild in livestock ruminants like epizootic hemorrhagic disease and blue tong virus (Araújo-Júnior et al., 2010; Ruder et al., 2015) in which 100% lethality has already been reported for brocket deer in captivity (Baldini et al., 2018). The presence of domestic dogs in protected areas was identified as a threat to several mammal species including deer (Lessa et al., 2016). Free-roaming domestic dogs’ should especially affect small forest deer, as demonstrated for the pudu (Pudu puda) in Chile, where dog attacks were frequent and lethal, and dog presence shaped the species distribution in a landscape scale (Silva-Rodríguez and Sieving, 2012).
We would emphasize that methodological studies of the Cervidae family are more recent than most of the management plans analysed. This shows the divergence between practical needs and science production, as well as exposing the time lag between scientific publications, their assimilation by the community, and the revision of documents such as management plans. The literature criticizes the distance between academia and conservation professionals and managers, who do not always follow practices supported by scientific evidence (Pullin et al., 2004). On one hand, efforts are needed to consolidate information and make it accessible to professionals, while on the other hand, it is necessary to identify the practical needs that require research support (Laurance et al., 2012). In that regard, the empirical evaluation of identification methods, the production of technical and didactic material, and the offer of professional training could contribute to improving the scenario described here.
Other initiatives should help to improve the quality of identifications in inventories. Camera traps are becoming more technologically advanced and more popular (Caravaggi et al., 2017), and it is possible that this technique will be used in most future mammal inventories, thus improving identification of branch-antlered species and favouring primary data collection and population trends monitoring (Tobler et al., 2008). Molecular identification methodologies using non-invasive samples, as fecal DNA, are also increasingly being used. This approach allowed a wide distribution assessment regarding the two endemic and threatened deer species in the Atlantic Forest of Brazil, Argentina and Paraguay (Duarte et al., 2017; Oliveira et al., 2019) and was also proposed to access information on leporids (Rodrigues et al., 2020), canids and felids (Rodríguez-Castro et al., 2020) in South America. Despite the need for a laboratory facility that involves costs and expertise, the development of simple methods using routine procedures (PCR and electrophoresis; see González et al., 2009), coupled with a reduction in the cost involved (Stein, 2010), should represent a step forward in the acquisition of reliable information. Finally, a closer relationship between professionals in the field and taxon specialists should be established, enabling a second opinion to be obtained for collected records, which should also contribute to more accurate identification.
Despite the ecological importance of deer and the threatened status of Neotropical species, our work shows that unsuitable methods have been used systematically in the identification of this group in Brazil and it is particular problematic regarding the identification of forest deer of the genus Mazama. Other countries, such as Peru, Equator, and Colombia, may face the same reality once it is no difficult to detect MPs and reports lacking species presence data, methodological information and using the same methods we found in Brazil (e.g. Zarate et al., 2018; Ministerio del Ambiente, 2015; SERNANP, 2014). The National System of Biodiversity Information of México, as an example, provides a database of species records that is limited to report detection method as “observed, reported, or collected” (CONABIO, 2021).
The problem of unreliable species identification should be of major concern for a wider group of Neotropical ungulates, certainly for the other five Mazama species distributed from México through Central America and Andean countries to the north of Argentina and Chile. Indirect detection methods such as tracks and faeces may also be inconsistent for pudu, huemul and even for tapirids and tayassuids species identification. The present problem should also be investigated in other tropical regions where similar small forest ungulates assemblies occur, like the duíkers (Cephalophinae; Bovidae) in Africa tropical forests and muntjacs (Muntiacinae; Cervidae) and Chevrotain (Tragulidae) in southeast Asia. These small forest ungulates share many ecological features (Geist et al., 2000) thus probably face the same challenges for species detection and identification like elusive behavior, visual and fecal morphological similarity, and even the presence of cryptic species (see Akomo-Okoue et al., 2015).
We conclude that greater scientific rigor is necessary, and inventories conducted by managers of protected areas should be of high quality, since they not only provide support for local management, but also form the basis of public policy on the conservation of threatened species worldwide. The call for more reliable information is particularly important nowadays when a lot of the data is collected by volunteers through citizen-science (Cohn, 2008) or made available through public databases (ex. Gen Bank, PortalBio, speciesLink, GBIF) and data papers (e.g. Lima et al., 2017), which do not always provide clear information regarding the identification methods used or the expertise of the data collectors. In this context, increasing the reliability of fauna surveys and continuing efforts to reduce biases associated with incorrect species identification should improve our capacity to evaluate the current state and trajectory of species populations and directing the investment of resources toward more assertive conservation actions.
FundingThis study and the authors were supported by The São Paulo State Foundation (FAPESP) under the projects: 15/25742-5, 17/02200-8, 17/07014-8; National Council for Scientific and Technological Development (CNPq) under project number 302368/2018-3; and the Coordination for the Improvement of Higher Education Personnel (CAPES).
Supplemental online material Species geographic distribution (A), a comparative image of Brazilian deer (B), the list of protected areas (C) and the occurrence records details (regarding species geographic distribution, number of records per species and biomes; (D) are available in Supplementary Material. The authors are solely responsible for the content and functionality of these materials and queries should be directed to the corresponding author.
The following is Supplementary data to this article: